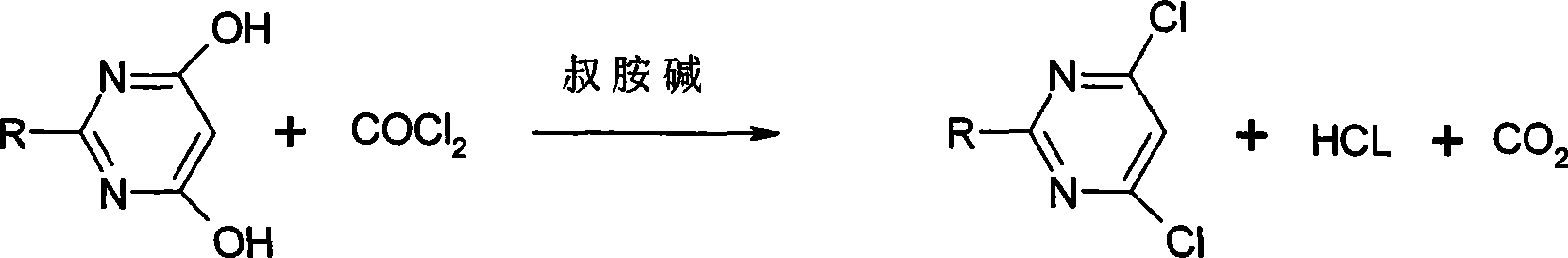
Method for producing substituted dichloropyrimidine
A technology of dichloropyrimidine and a production method, applied in 4 fields, can solve the problem of not solving the problem of phosphorus-containing wastewater discharge, and achieve the effects of protecting water resources, reducing costs, and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a well-ventilated fume hood, install a stirring, thermometer, condenser, and vent pipe on a 500-milliliter reaction bottle, and then prepare an exhaust gas absorption and scrubbing bottle, and put liquid alkali in the bottle with a concentration of 10%-20%. Absorb exhaust fumes. Put 15.8g of 2-methylthio-4,6-dihydroxypyrimidine (99%), 250ml of chlorobenzene and 2g of 1-methylimidazole into the flask successively, heat up and reflux, and pass through the airway tube at a rate of about 0.1L / min. Pass through phosgene, follow up with sampling, until high pressure liquid chromatography (HPLC) shows that dihydroxypyrimidine disappears, and it takes about 16 hours to complete. Stop the reaction, drive away excess phosgene with nitrogen, cool, filter, wash the filtrate with water until neutral, dry over anhydrous sodium sulfate and remove chlorobenzene to obtain 18.0 g of 2-methylthio-4,6-dichloropyrimidine , content 96.0%.
Embodiment 2
[0031] In a well-ventilated fume hood, install a stirring, thermometer, condenser, and vent pipe on a 500-milliliter reaction bottle, and then prepare an exhaust gas absorption and scrubbing bottle, and put liquid alkali in the bottle with a concentration of 10%-20%. Absorb exhaust fumes. In the flask, drop into 14.2g 2-methoxy group-4,6-dihydroxypyrimidine (99%), 200ml of 2-dichloroethane and N,N-dimethylaniline 19g successively, heat up and reflux, with about Introduce phosgene through the airway at a rate of 0.1 L / min, take samples and follow up until HPLC shows that dihydroxypyrimidine disappears, which takes about 15 hours to complete. Stop the reaction, drive away the excess phosgene with nitrogen, cool down, remove the solvent dichloroethane, add 300ml of toluene, wash with 100ml of water, separate layers, remove the toluene after drying with anhydrous sodium sulfate, and obtain 2-methoxy - 16.5 g of 4,6-dichloropyrimidine. Content 95.5%.
Embodiment 3
[0033] In a well-ventilated fume hood, install a stirring, thermometer, condenser, and vent pipe on a 500-milliliter reaction bottle, and then prepare an exhaust gas absorption and scrubbing bottle, and put liquid alkali in the bottle with a concentration of 10%-20%. Absorb exhaust fumes. 14.0g of 2-ethyl-4,6-dihydroxypyrimidine (99%), 300ml of dichloromethane and 6.4g of 4-dimethylaminopyridine were successively put into the flask, the temperature was raised to reflux, and the The airway was fed with phosgene, and samples were taken and tracked until HPLC showed that the dihydroxypyrimidine disappeared, which took about 10 hours to complete. Stop the reaction, drive away excess phosgene with nitrogen, cool, filter, wash the filtrate with water until neutral, dry over anhydrous sodium sulfate and remove dichloromethane to obtain 15.6g of 2-ethyl-4,6-dichloropyrimidine . The content is 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com