Fe-based bulk amorphous alloy material and method of producing the same
An amorphous alloy and bulk technology, which is applied in the field of iron-based bulk amorphous alloy materials and their preparation, can solve problems such as inability to widely use engineering materials, and achieve the effects of high hardness, simple process and strong glass forming ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
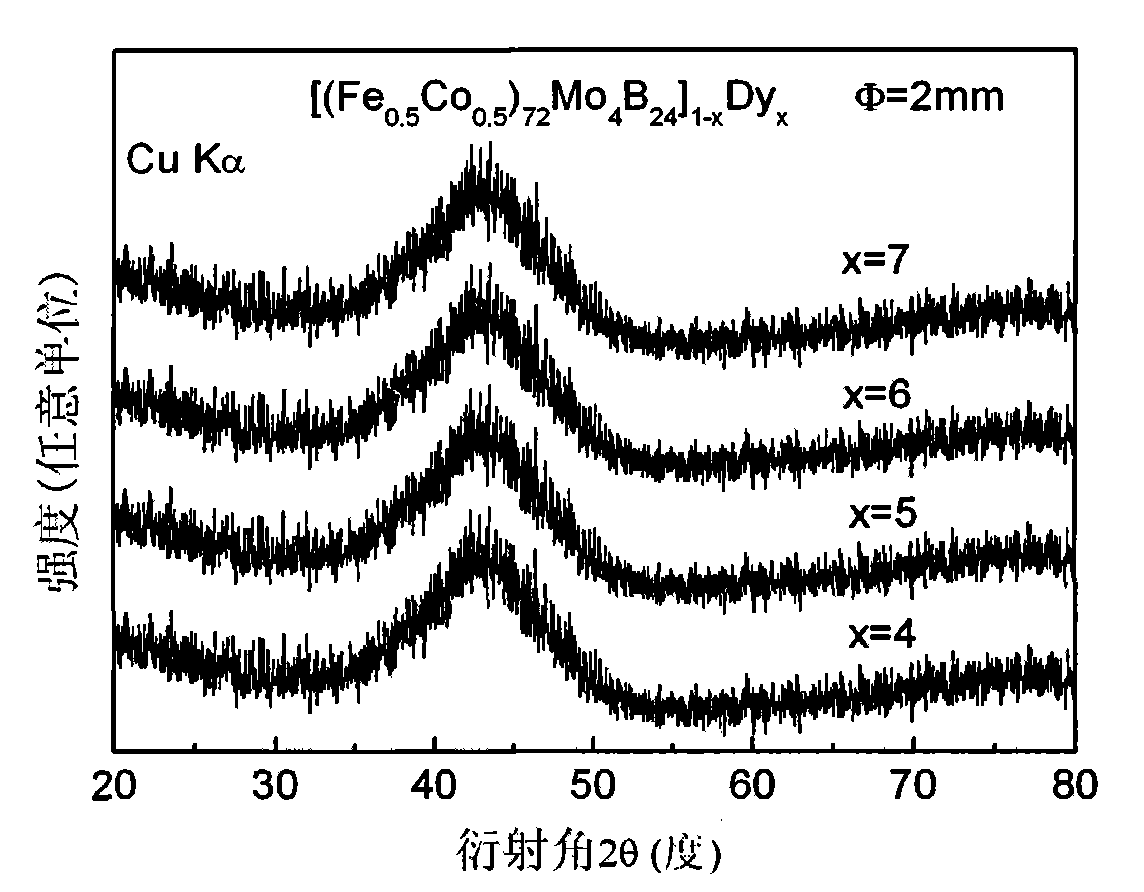
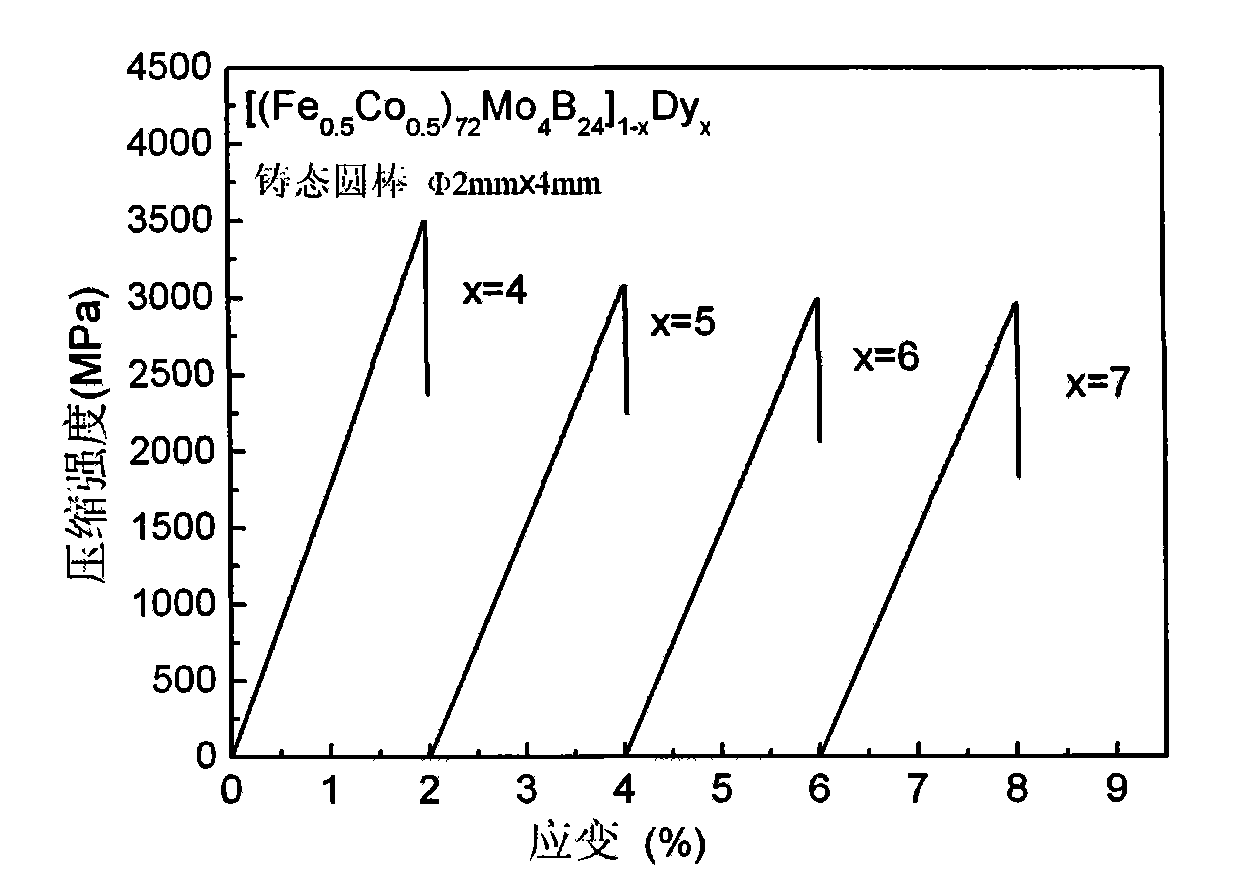
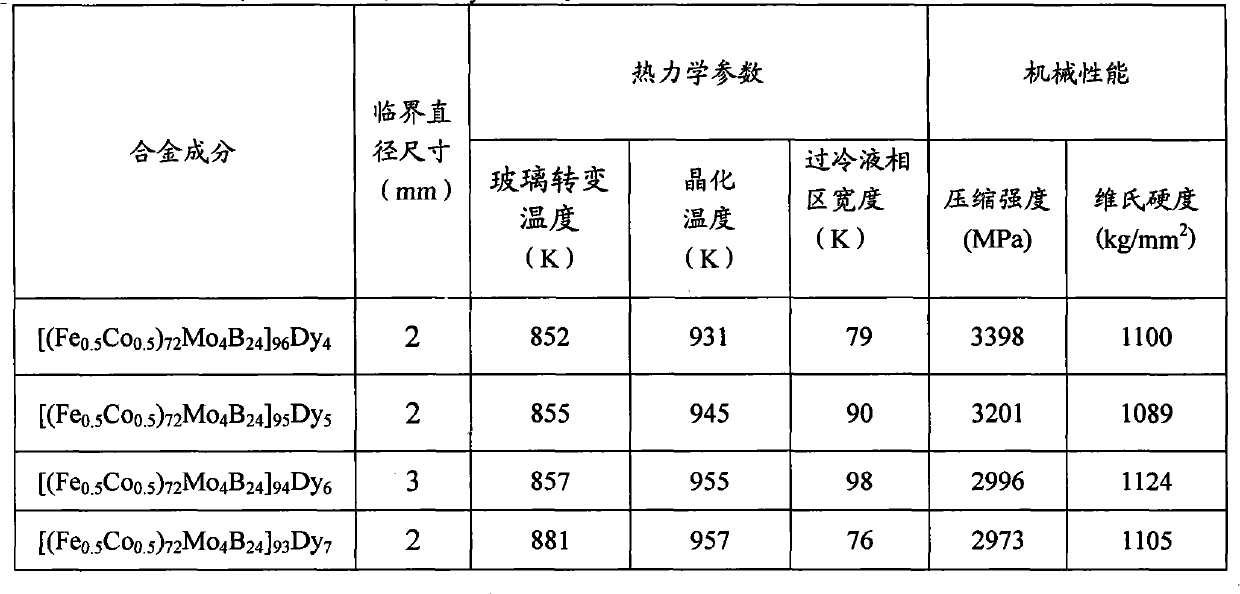
[0016] Embodiment 1: preparation [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 96 Dy 4 Amorphous Alloy Rods
[0017] Fe, Co, Mo, B and Dy elements according to [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 96 Dy 4 After the alloy atomic percentage is converted into mass percentage, accurately weigh Fe with a purity of 99.6%, Co with a purity of 99.9%, Mo with a purity of 99.8%, Dy with a purity of 99.9% and FeB alloy, and put the quartz in the induction furnace tube, to be evacuated to 4.0×10 -3 Pa, filled with high-purity argon protection, adjust the current from small to large, induction heating until the sample melts. Repeated smelting for 9 times to obtain evenly mixed [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 96 Dy 4 Master alloy ingots. will get [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 96 Dy 4 The master alloy ingot was broken into small pieces and ultrasonically cleaned in alcohol. Then put it into a quartz tube with an open lower end and a hole diameter of 0.5mm, and draw a vacuum ...
Embodiment 2
[0018] Embodiment 2: preparation [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 95 Dy 5 Amorphous Alloy Rods
[0019] Fe, Co, Mo, B and Dy elements according to [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 95 Dy 5After the alloy atomic percentage is converted into mass percentage, accurately weigh Fe with a purity of 99.6%, Co with a purity of 99.9%, Mo with a purity of 99.8%, Dy with a purity of 99.9% and FeB alloy, and put the quartz in the induction furnace tube. To be vacuumed to 4.0×10 -3 Pa, filled with high-purity argon protection, adjust the current from small to large, induction heating until the sample melts. Repeated smelting 10 times to obtain a well-mixed [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 95 Dy 5 Master alloy ingots. will get [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 95 Dy 5 The master alloy ingot was broken into small pieces and ultrasonically cleaned in alcohol. Then put it into a quartz tube with an open lower end and a hole diameter of 0.4 mm, and draw a vacuum to ...
Embodiment 3
[0020] Embodiment 3: preparation [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 94 Dy 6 Amorphous Alloy Rods
[0021] Fe, Co, Mo, B and Dy elements according to [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 94 Dy 6 After the alloy atomic percentage is converted into mass percentage, accurately weigh Fe with a purity of 99.6%, Co with a purity of 99.9%, Mo with a purity of 99.8%, Dy with a purity of 99.9% and FeB alloy, and put the quartz in the induction furnace tube. To be vacuumed to 4.0×10 -3 Pa, filled with high-purity argon protection, adjust the current from small to large, induction heating until the sample melts. Repeated smelting 6 times to obtain evenly mixed [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 94 Dy 6 Master alloy ingots. will get [(Fe 0.5 co 0.5 ) 72 Mo 4 B 24 ] 94 Dy 6 The master alloy ingot was broken into small pieces and ultrasonically cleaned in alcohol. Then put it into a quartz tube with an open lower end and a hole diameter of 0.4 mm, and draw a vacuum to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com