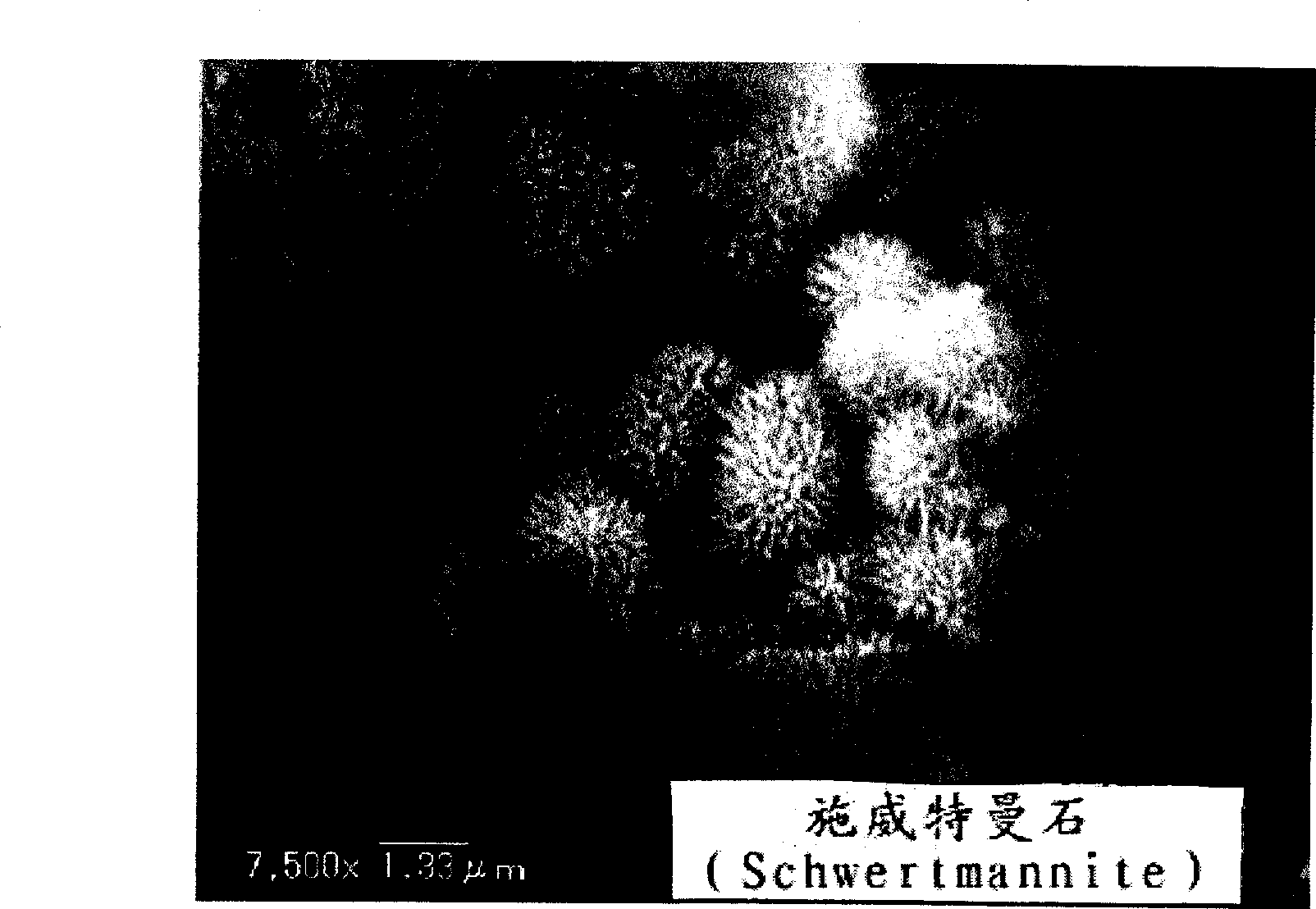
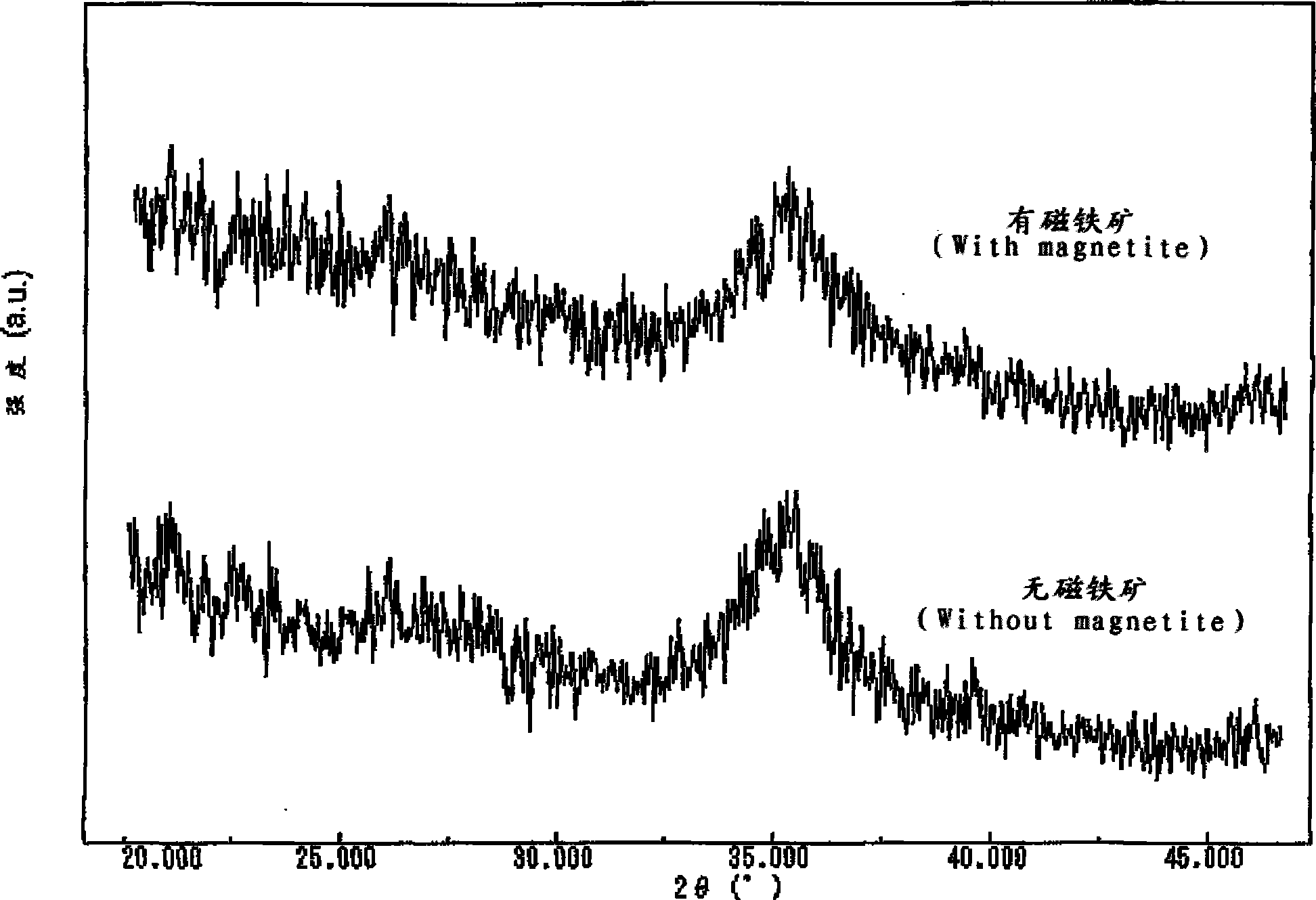
Magnetic chemical absorber, process for producing the same, method of regenerating the same, and method of treating waste liquid
A technology for chemical absorption and waste liquid treatment, applied in chemical instruments and methods, adsorbed water/sewage treatment, solid adsorbent liquid separation, etc. Separation, usage reduction, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] A magnetic chemical absorbent was produced by the production method shown below.
[0094] First, by adding ferrous chloride (FeCl 2 ) and ferric chloride (FeCl 3 ) mixture (FeCl 2 :FeCl 3 The molar ratio is 1:2) and slowly add diluted ammonia solution to adjust the pH to 8 to precipitate magnetite particles with an average particle diameter of about 10-100 nm. Next, magnetite fine particles are separated from solid and liquid by magnetic separation, and then washed several times until the pH value is 7, and the magnetite fine particles are ready.
[0095] Then, dissolve 25g of Fe in 500ml of distilled water 2 (SO 4 ) 3 ·5H 2 O to obtain an aqueous solution, which was heated to 60° C. and stirred slowly for 10 minutes (heating step).
[0096] Next, to the aqueous solution maintained at 60°C, 10 ml of a suspension in which 3 g of the magnetite microparticles were suspended in 1000 ml of distilled water was added, and then added to the aqueous solution maintained a...
Embodiment 2
[0150] ready as Figure 14 The experimental setup shown in the schematic diagram. In this experimental device, while the glass tube 2 is set in the magnetic field 1 from bottom to top, a magnetic filter 3 made of ferromagnetic wires is set at the position where the magnetic field gradient in the glass tube 2 is the largest.
[0151] And, apply the magnetic field 1 of intensity shown in Table 1, the 100ml waste water that will be suspended with the magnetic chemical absorber that embodiment 1 provides, flow down from above with 3~4 seconds in described glass tube 2, make this waste water flow from magnetic The top of the filter 3 passes downward. At this time, the mass ratio of the magnetic chemical absorbent that can be magnetically separated from wastewater was investigated, and the results are shown in Table 1.
[0152] For comparison, the Schwittmannite alone obtained in Comparative Example 1 was also investigated in the same manner. The results are shown in Table 1 toge...
Embodiment 3
[0158] In the waste water whose initial P concentration is 20mg / L and the pH value is 7, add the magnetic chemical absorbent provided by Example 1 with an addition ratio of 1g / L, stir for 3 hours, and make the magnetic chemical absorbent absorb the P in the wastewater. p. At this time, the P absorption rate of the new product state of the magnetic chemical absorbent provided in Example 1 was investigated, and as shown in Table 2, it was 99.7%.
[0159] It should be noted that this P absorptivity is obtained by a photometer.
[0160] In addition, the magnetic chemical absorbent provided in Example 1 that absorbs P in saturation can be put into a 0.05 mol / L sulfuric acid aqueous solution and stirred for 10 minutes, so that the magnetic chemical absorbent can be regenerated for the first time. Afterwards, the regenerated magnetic chemical absorbent was separated by filtration, washed with deionized water to reach a pH value of 7, and dried.
[0161] Furthermore, the P absorptio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com