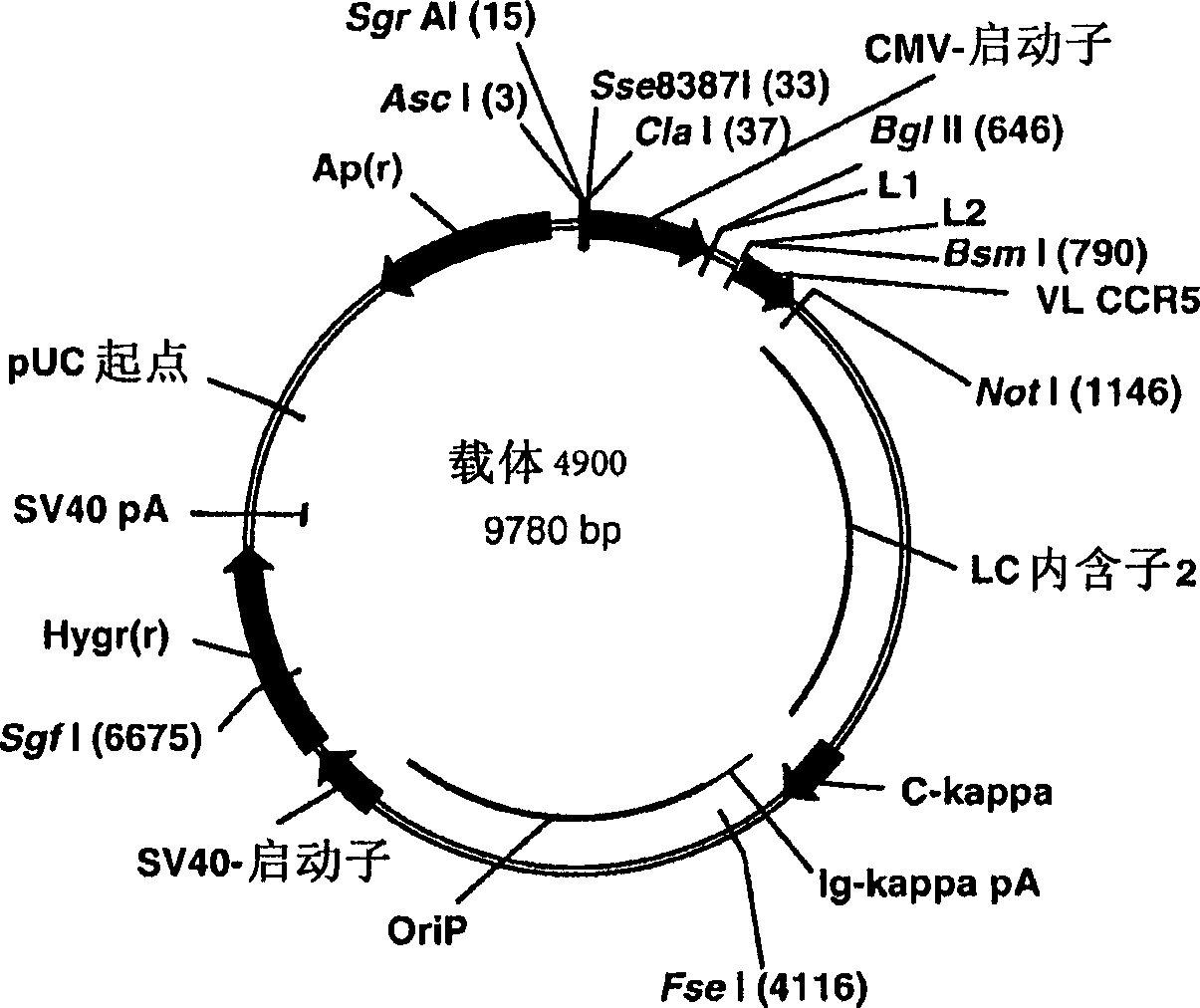
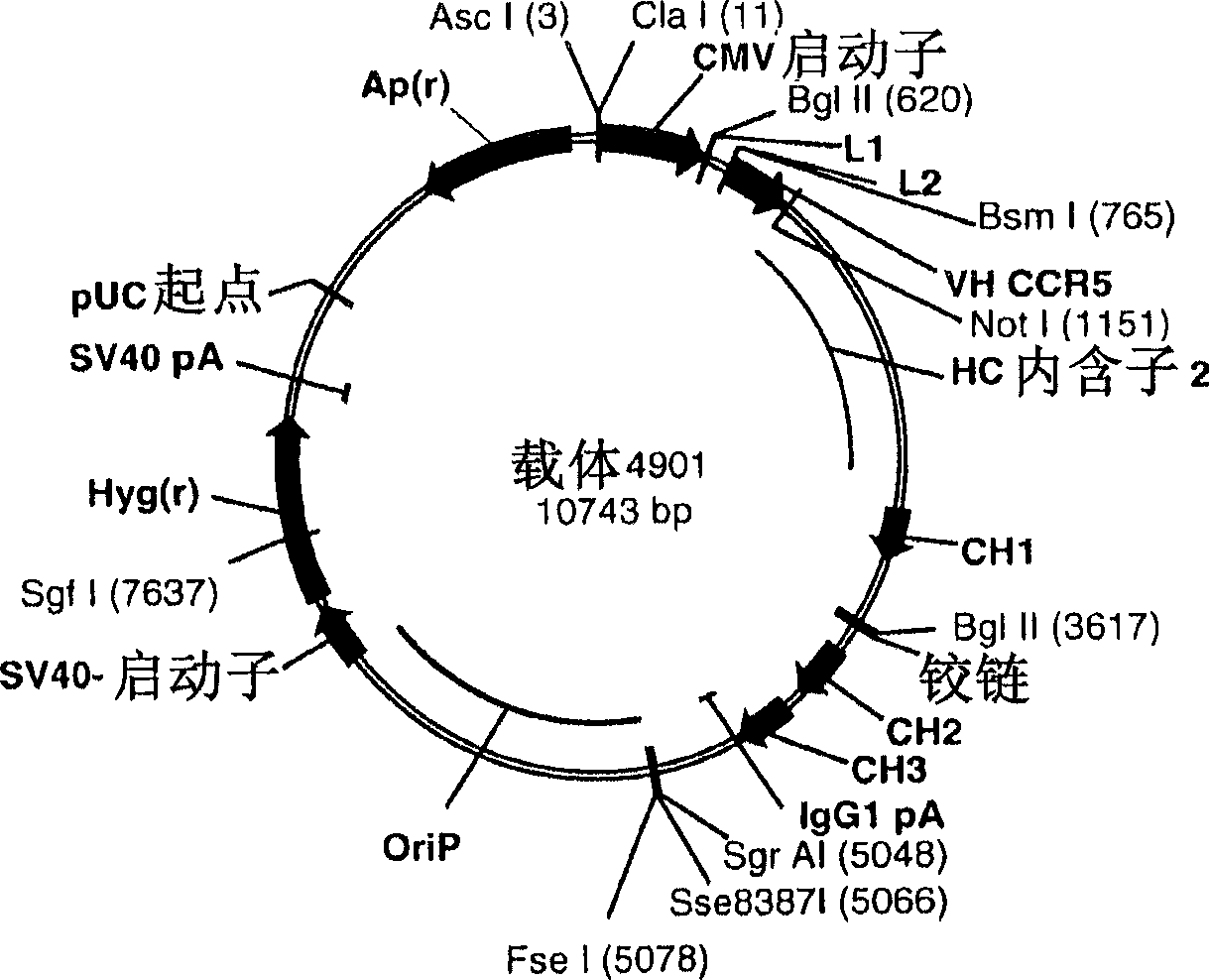
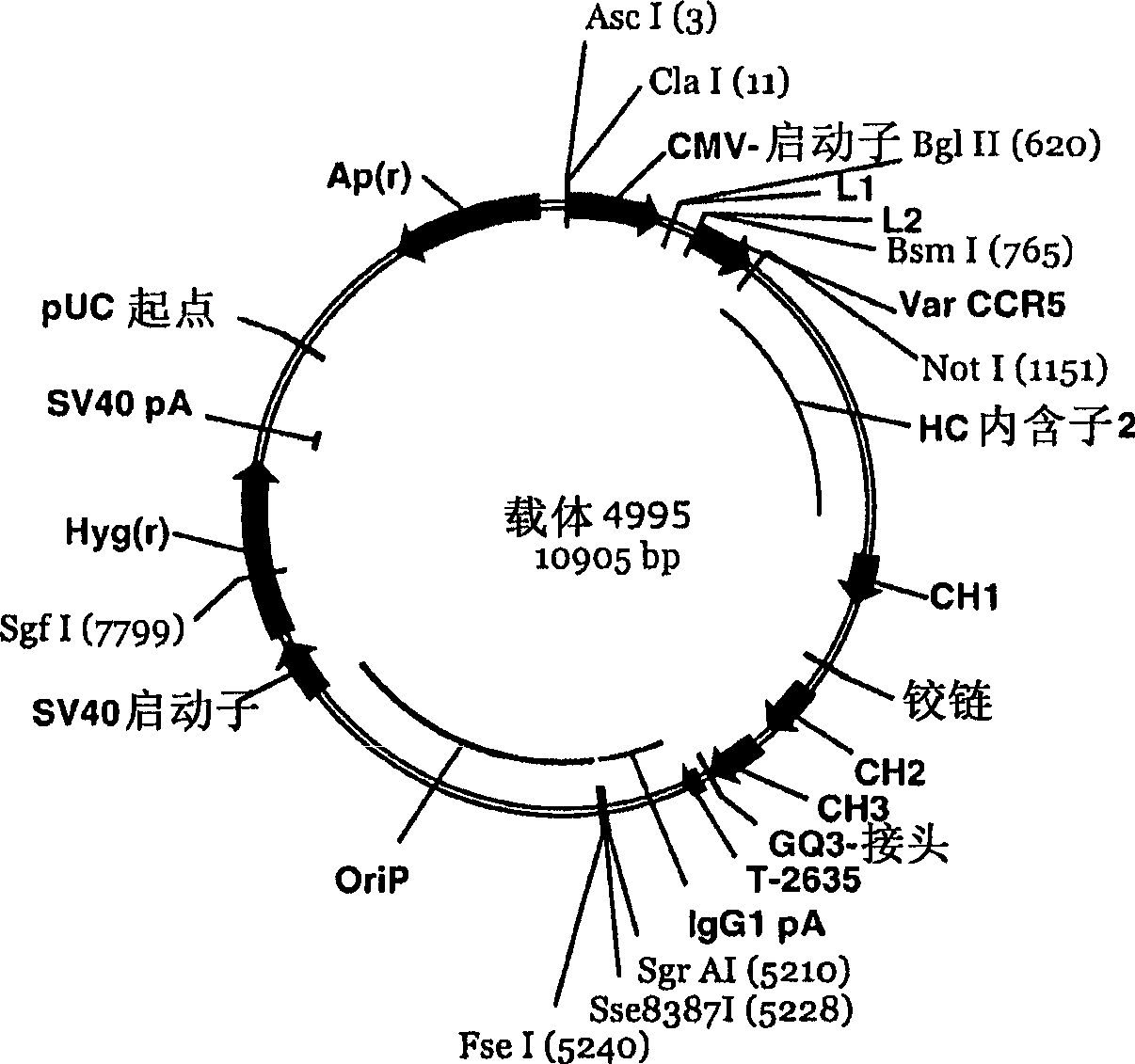
A conjugate of an antibody against CCR5 and an antifusogenic peptide
A technology of -CCR5 and conjugates, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, non-active ingredients of polymer compounds, peptides, etc., can solve the problem that human health is not necessary
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Preparation of anti-CCR5 antibody expression plasmid
[0242] will encode the anti-CCR5 antibody light chain variable domain (V L ) and human κ-light chain constant domain (C L ) gene fragment combination, will also be about the anti-CCR5 antibody heavy chain variable domain (V H ) and human γ1-heavy chain constant domain (C H 1-Hinge-C H 2-C H 3) The combination of gene fragments.
[0243] In the case of mAb CCR5 of SEQ ID NO: 63 / 64, the heavy and light chain variable domains were derived from mouse antibodies and the heavy and light chain constant domains were derived from human antibodies (C-κ and IgG1) .
[0244] Subsequently, the gene fragment encoding the complete anti-CCR5 antibody light chain is linked at the N-terminus and / or C-terminus to the nucleic acid encoding the anti-fusion peptide, including the linker sequence, and / or the encoding complete anti-CCR5 antibody The gene segment of the heavy chain is linked at the N-terminus and / or C-t...
Embodiment 2
[0300] Preparation of the final anti-CCR5 antibody expression plasmid
[0301] Using known recombination methods and techniques, by connecting the nucleic acid fragments, a fusion gene (heavy chain and / or light chain antibody fusion gene) is assembled, the fusion gene comprises mAb CCR5 gene fragment, optional linker gene fragment and antifusion peptide gene fragments. Nucleic acid sequences encoding the peptide linker and the anti-fusion polypeptide were separately synthesized by chemical synthesis, and then ligated into an E. coli plasmid for amplification. The nucleic acid sequence of the subclones was verified by DNA sequencing.
Embodiment 3
[0303] Transient expression of immunoglobulins and immunoglobulin variants in HEK293EBNA cells
[0304] Recombinant anti-CCR5 antibody and anti-CCR5 antibody-variants were passed through adherently grown HEK293-EBNA cells (human embryonic kidney cell line 293 expressing Epstein-Barr virus nuclear antigen; American Type Culture Collection ATCC # CRL-10852 ), the cells were grown in supplemented with 10% ultra-low IgG FCS (fetal calf serum, Gibco), 2 mM glutamine (Gibco), 1% volume / volume (v / v) non-essential amino acids (Gibco) and 250 μg / ml G418 (Roche Molecular Biochemicals) in DMEM (Dulbecco's modified Eagle medium, Gibco). For transfection, FuGENE TM 6 Transfection reagent (Roche Molecular Biochemicals) was used in a reagent (μl):DNA (μg) ratio of 3:1-6:1. The light and heavy chains comprising the anti-fusion peptide-anti-CCR5 antibody conjugate light and heavy chains were expressed from two different plasmids, respectively, using a 1:2-2:1 light chain-encoding plasm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com