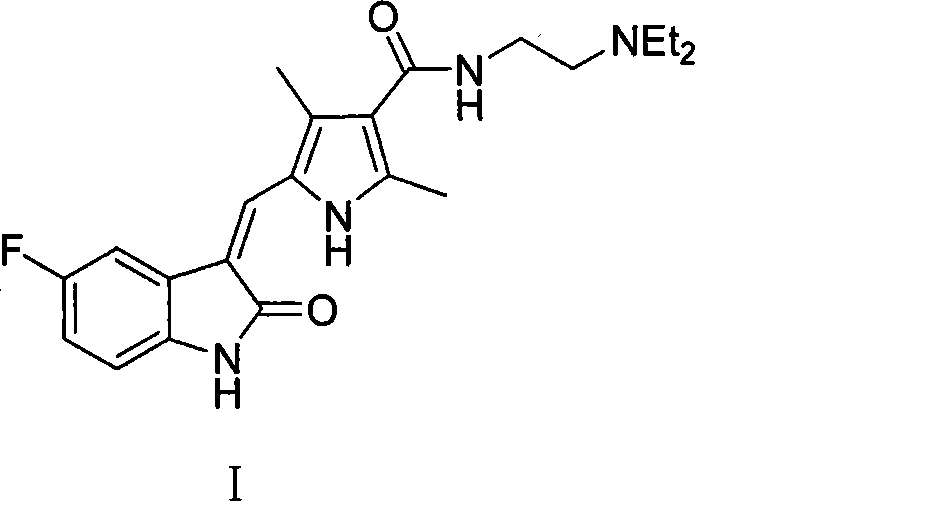
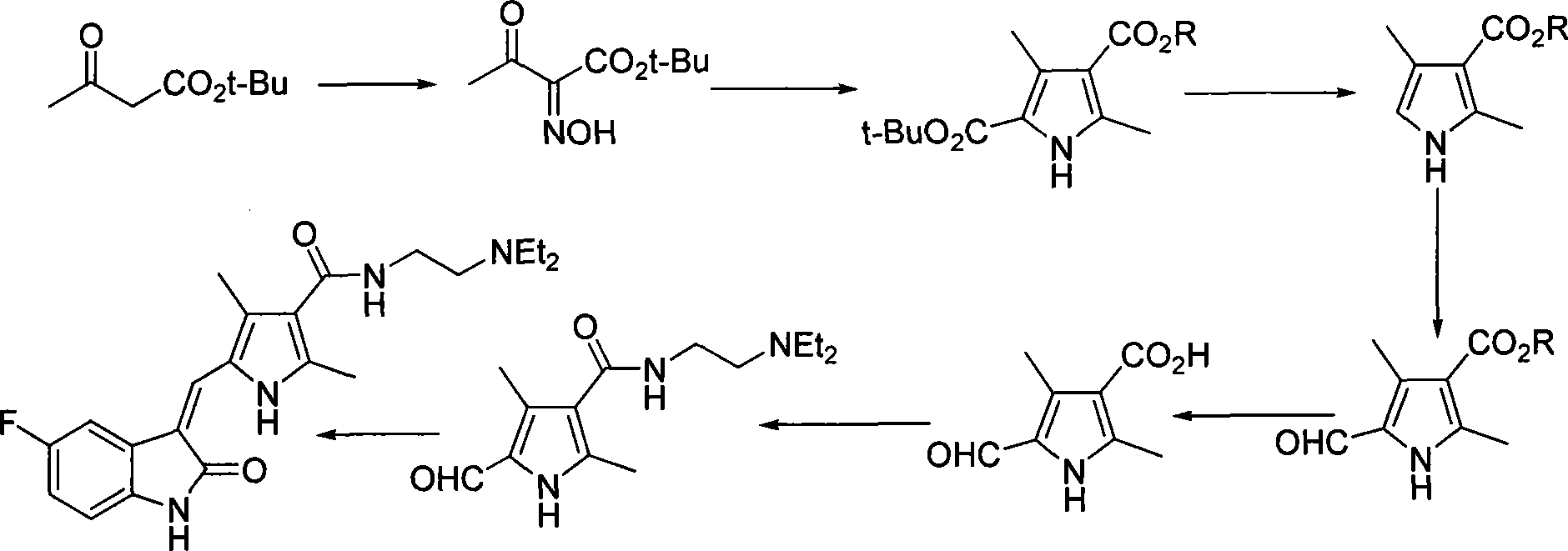
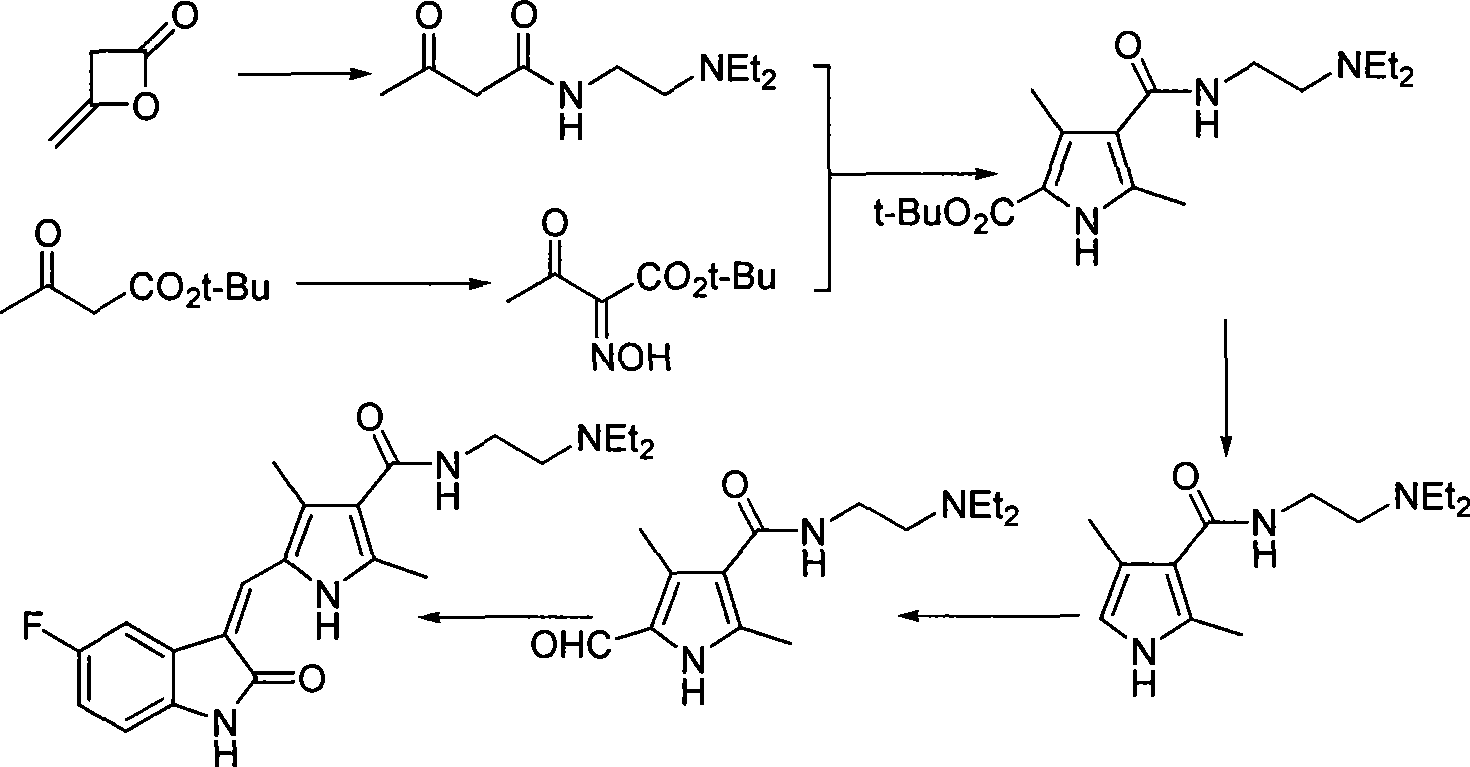
Process for synthesizing sunitinib
A synthesis method, sunitinib technology, applied in the direction of organic chemistry, can solve the problems of increasing the difficulty of purification, and achieve the effects of easy crystallization and purification, simplified purification methods, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Add 7.5 liters of acetic acid and 5.0 kg of tert-butyl acetoacetate into a 25-liter plastic bucket, add dropwise a solution of 2.24 kg of sodium nitrite dissolved in 3.5 liters of water at 0 degrees, control the temperature not to exceed 5 degrees, add 3 liters of water after dropping, and keep warm for reaction After 30 minutes, remove the cold bath, and slowly heat up the reaction. After the reaction, add 5 liters of water, carefully neutralize it with solid sodium bicarbonate until it is nearly neutral, and finally adjust the pH to 7-8 with a small amount of 4N sodium hydroxide solution. Extracted with ethyl ester, dried and concentrated to obtain about 5.6kg of liquid, which was directly used in the next reaction.
[0029] In 25 liters of plastic barrels, add 2.8kg of the product of the previous step, 3kg of ethyl acetoacetate and 10 liters of acetic acid, add the mixture of 3.4kg of zinc powder and 3.4kg of sodium acetate in batches, control the feeding ...
Embodiment 2
[0045]
[0046] Add 1.5 liters of acetic acid and 1.0 kg of tert-butyl acetoacetate to a 5-liter four-neck bottle, add dropwise a solution of 0.5 kg of sodium nitrite dissolved in 2 liters of water at 0 degrees, control the temperature not to exceed 5 degrees, add 1 liter of water after dropping, and keep warm React for 30 minutes, remove the cold bath, and slowly heat up the reaction. After the reaction, add 2 liters of water, carefully neutralize it with solid sodium bicarbonate to near neutrality, and finally adjust the pH to 7-8 with a small amount of 4N sodium hydroxide solution. Extracted with ethyl acetate, dried and concentrated to obtain about 1.2kg of liquid, which was directly used in the next reaction.
[0047] In a 5-liter four-necked bottle, add 1.2kg of the product from the previous step, 1kg of propyl acetoacetate and 2 liters of acetic acid, add a mixture of 0.8kg of zinc powder and 0.8kg of sodium acetate in batches, and control the feeding speed so that th...
Embodiment 3
[0054]
[0055] Add 100 liters of acetic acid and 50 kg of tert-butyl acetoacetate to a 250-liter kettle, add dropwise a solution of 25 kg of sodium nitrite dissolved in 35 liters of water at 0°C, control the temperature not to exceed 5°C, add 30 liters of water after dropping, and keep the temperature for 30 minutes. Remove the cold bath, slowly heat up the reaction, after the reaction, add 50 liters of water, carefully neutralize to near neutral with solid sodium bicarbonate, finally adjust the pH to 7-8 with a small amount of 4N sodium hydroxide solution, and extract with ethyl acetate , dried, and concentrated to obtain about 56kg of liquid, which was directly used in the next reaction.
[0056] In the 250 liter kettle, add 30kg of the product of the previous step, 3kg of methyl acetoacetate and 150 liters of acetic acid, add the mixture of 50kg of zinc powder and 50kg of sodium acetate in batches, control the feed rate, so that the temperature is no more than 85 degrees...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com