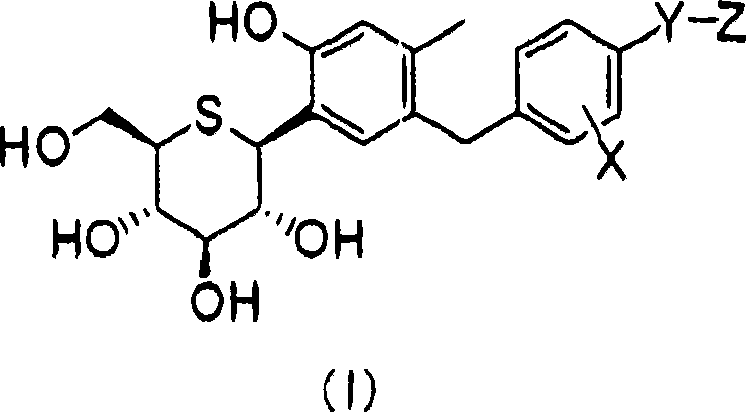
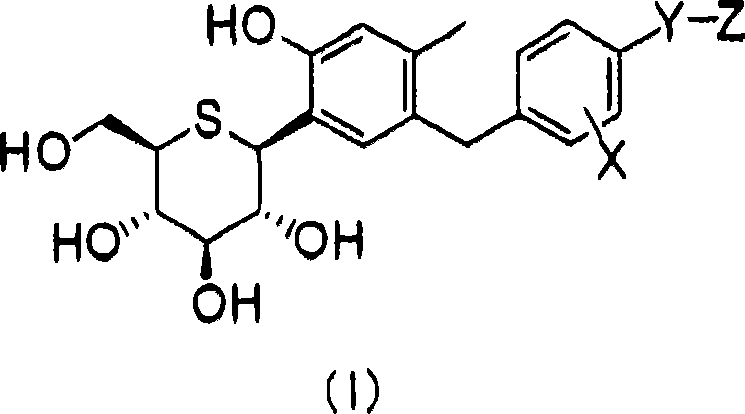
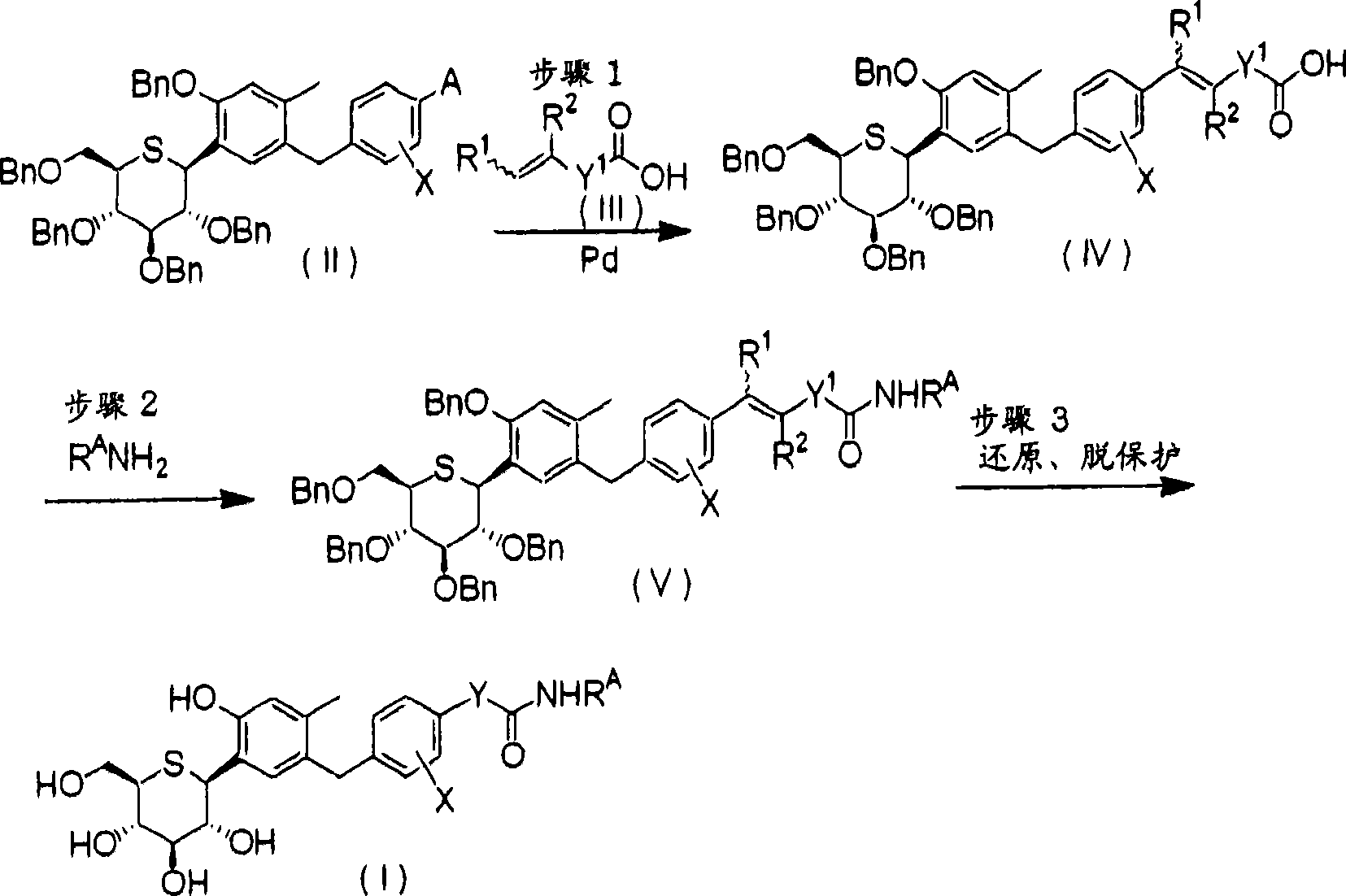
C-phenyl 1-thioglucitol compound
A technology of thiosorbitol and compounds, applied in the fields of carbocyclic sugars, metabolic diseases, organic chemistry, etc., can solve the problem of strong inhibition without reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] The preparation method of intermediate (II)
[0107] The production methods of intermediates (II) and (VIII) necessary for the production of the compound (I) of the present invention are shown below. Among them, D 1 Indicates Li or MgBr, and other symbols have the same meaning as above.
[0108] 【Chemical 6】
[0109]
[0110] (17) Step 17 (Coupling)
[0111] Aryllithium reagents can be prepared by using organometallic reagents such as n-butyllithium, sec-butyllithium, and tert-butyllithium for intermediate compound (XXII). Compound (XXIII) can be obtained by adding thiolactone (XVI) thereto. The solvent used for the reaction at this time may, for example, be tetrahydrofuran, diethyl ether or toluene. The reaction temperature is -80°C to room temperature, preferably -78°C to -25°C.
[0112] (18) Step 18 (acid hydrolysis)
[0113] Compound (VIII) can be produced by hydrolyzing the acetal group in compound (XXIII) with hydrochloric acid, p-toluenesulfonic acid mo...
Embodiment
[0151] The present invention will be further described in detail below in conjunction with reference examples, examples and test examples.
reference example 1
[0153] Preparation of 2,3,4,6-tetra-O-benzyl-5-thio-D-glucono-1,5-lactone (compound (XVI))
[0154] 【chemical 8】
[0155]
[0156] (1) Preparation of tetrahydro-2H-pyran-2-yl 2,3,4,6-tetra-O-acetyl-5-thio-D-glucopyranose
[0157] To a solution of 2,3,4,6-tetra-O-acetyl-5-thio-D-glucopyranose (2.0 g, 5.49 mmoL) in chloroform (40 mL), add 3,4-dihydro-2H -pyran (1.5 mL, 16.5 mmoL) and p-toluenesulfonic acid monohydrate (104 mg, 0.549 mmoL), stirred at room temperature for 1 hour. Saturated aqueous sodium bicarbonate solution was added to the reaction liquid, followed by extraction with chloroform, and the organic layer was washed with saturated brine, and dried over anhydrous magnesium sulfate. After the desiccant was filtered off, the solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate=1:1) to obtain the title compound (2.56 g) as a light yellow amorphous substance.
[0158] (2) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com