Duplex metal phosphide catalyst for selective hydrogenation and olefin hydrocarbon removal as well as preparation method thereof
A catalyst and phosphide technology, applied in the field of solid catalyst and its preparation for selective hydrogenation of a small amount of olefins in aromatics, achieving good application prospects and high selective hydrogenation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of TiO by coprecipitation method 2 -Ni 2 P catalyst precursor. 3.90 grams of nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) dissolved in 15 mL of deionized water, 2.70 g of titanium tetrachloride (TiCl 4 ) was dissolved in 100mL ethanol, and an appropriate amount of TiCl 4 Add the ethanol solution into the nickel nitrate solution and stir well. Added TiCl 4 The amount of ethanol solution is determined according to the total Ti / Ni molar ratio. Then 1.77 grams of diamine hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was dissolved in 10mL deionized water and added dropwise to the above TiCl-containing 4 In the nickel nitrate solution in ethanol solution, a precipitate is formed. The resulting mixture was evaporated to dryness with stirring, the slurry was dried at 120° C. for 12 hours, and then calcined at 500° C. for 3 hours to obtain a catalyst precursor.
Embodiment 2
[0018] Preparation of CeO by impregnation method 2 -Ni 2 P catalyst precursor.
[0019] First, unsupported Ni was prepared by co-precipitation method 2 P catalyst precursor. Weigh 3.90 grams of nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) was dissolved in 15mL deionized water, 1.77 grams of diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was dissolved in 10 mL of deionized water. Then the diammonium hydrogen phosphate solution was added dropwise to the nickel nitrate solution to form a precipitate. The water was evaporated to dryness, and the solid product was dried at 120°C for 12 hours, and then calcined at 500°C for 3 hours to obtain Ni 2 P precursor.
[0020] Ni will be produced 2 After the P precursor is finely ground, it is added to the metered Ce(NO 3 ) 3 ·6H 2 O aqueous solution (the concentration is determined by the catalyst Ce / Ni molar ratio), impregnated for 12 hours, then dried at 120°C for 12 hours, and calcined at 500°C for 3 hours to obtain CeO-containi...
Embodiment 3
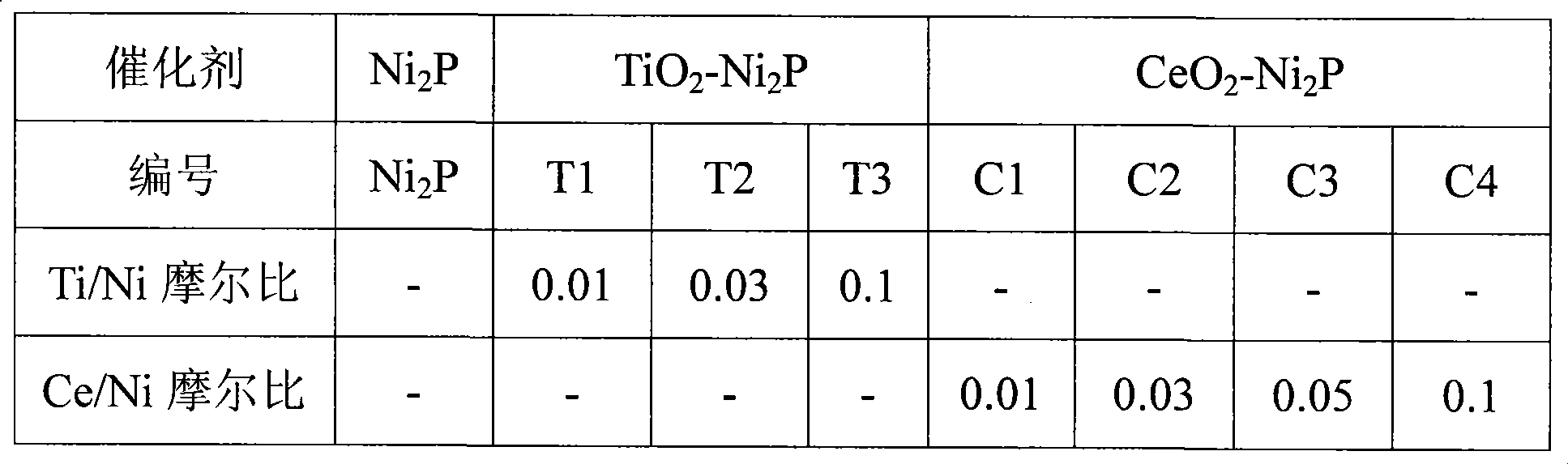
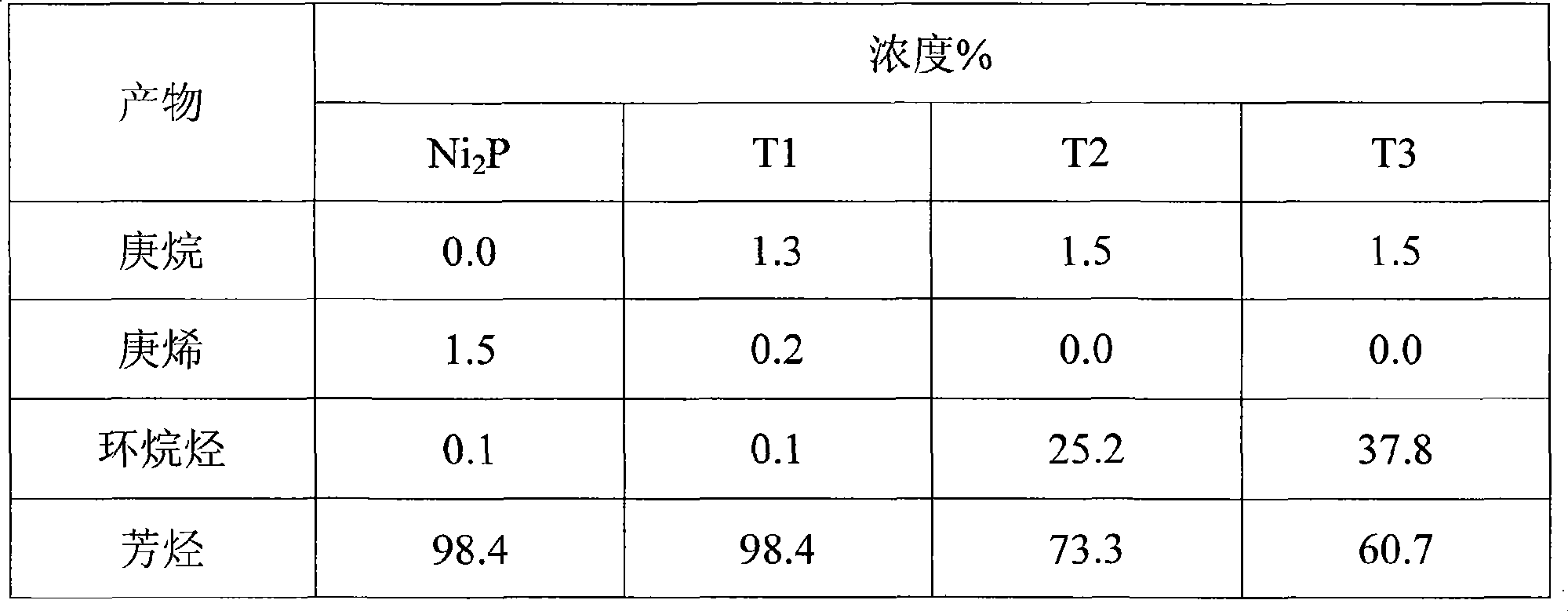
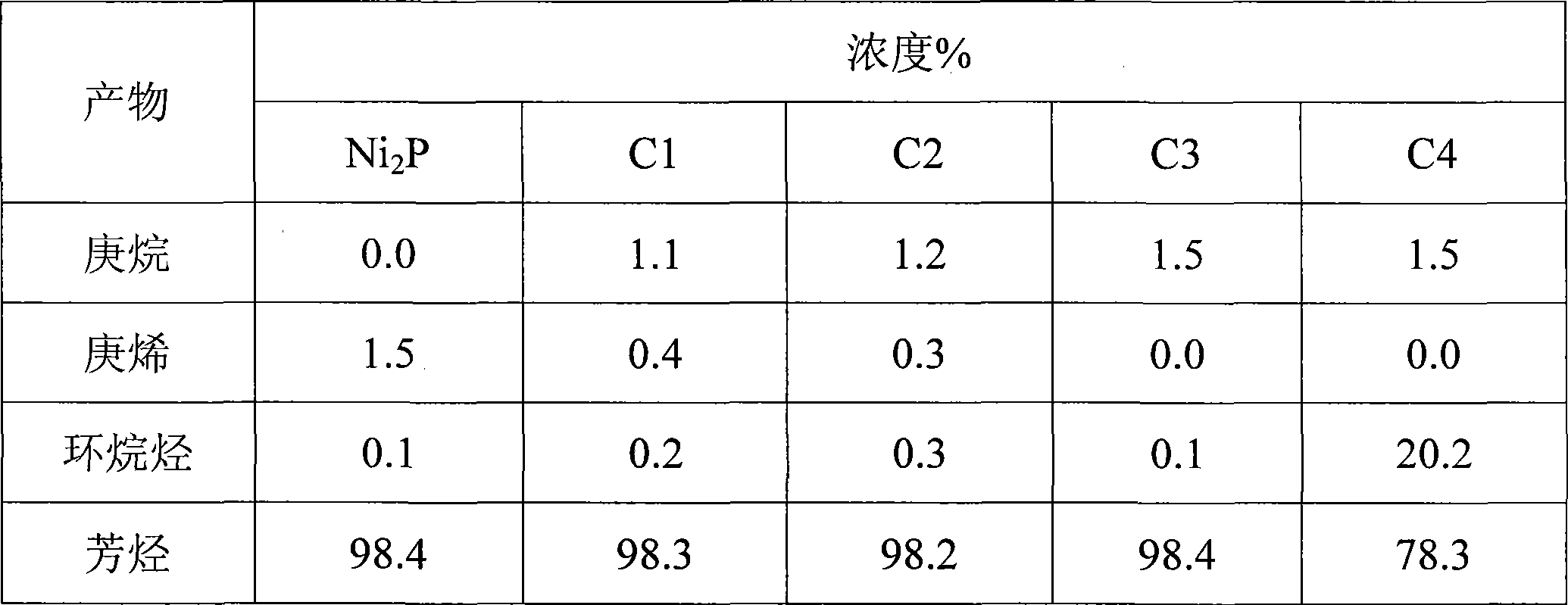
[0022] Weigh 0.5 g of the catalyst precursor in Example 1 or 2, and put it into a fixed-bed reactor with an inner diameter of 8 mm. In a hydrogen atmosphere, raise the temperature from room temperature to 400°C at a rate of 2°C / min, then raise it to 500°C at a rate of 1°C / min, keep it for 2 hours, and then cool down to the reaction temperature naturally to prepare a bimetallic phosphide catalyst. The gas flow rate is 200mL / min, and the pressure is 1MPa. Table 1 lists the catalysts prepared by the method of the present invention.
[0023] Table 1 Catalyst prepared by the present invention
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com