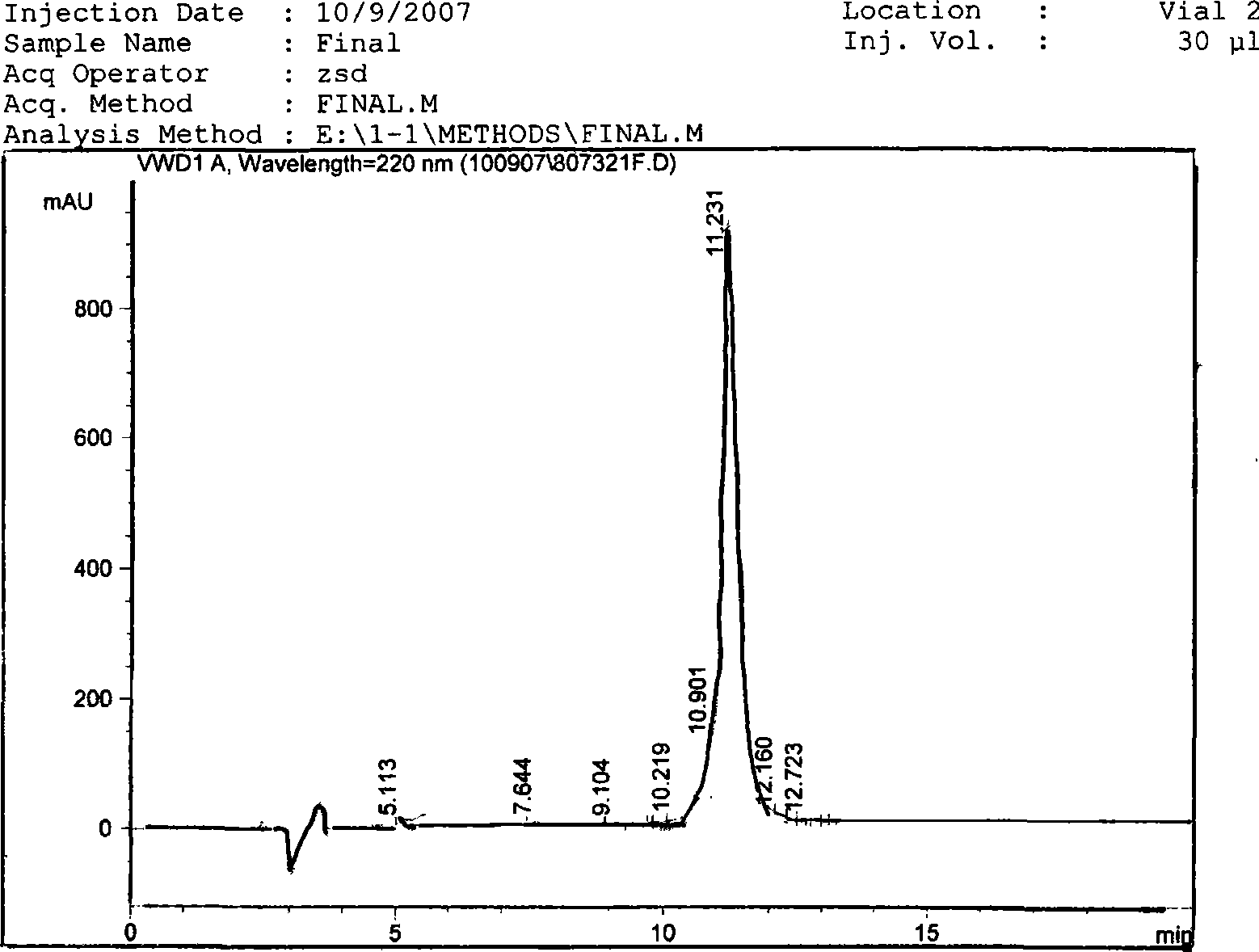
Trichosanthin protein derived peptide and use thereof
A technology of trichosanthin and derived peptides, which is applied in the direction of peptide/protein components, peptides, medical preparations containing active ingredients, etc., and can solve problems such as toxic reactions, destruction of the complete structure of epitopes, and constant cleavage points
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of toxic peptide (p14), inhibitory peptide (p18), and inhibitory peptide p18 tetramer
[0031] (1) Materials
[0032] Solid phase extraction cartridge (Waters company); Fluorenemethoxycarbonyl (Fmoc) protected amino acid (Merck company); 1-Oxo-3-bisdimethylaminocarbonyl benzotriazole boron tetrafluoride salt (TBTU), 1-Hydroxybenzotriazole (HOBT) (ABI company); linear polylysine, DIEA, trifluoroacetic acid (TFA) (Sigma company); G-10, G-25 gel (Pharmacia company); Acetonitrile (Fisher Corporation).
[0033] (2) Method
[0034] 1. Synthesis of Linear Peptides
[0035]0.5 g of PAC-PEG-PS resin (degree of substitution 0.2 mmol / g) was soaked in N-N dimethylformamide (DMF) for 30 min for activation. The activated resin was linked to the first amino acid by the symmetrical anhydride method. Weigh 195 mg of the amino acid to be linked, add 1.5 ml of dichloromethane (DCM) and 3 drops of DMF to dissolve it, add 275 mL of 1 mol / L dicyclohexylcarbodiimide (DCC) / N-m...
Embodiment 2
[0049] Trypan blue exclusion test and in vitro apoptosis induction test (1) Trypan blue test
[0050] This experiment is a simple method to detect whether the cell membrane of the cell to be tested is ruptured, that is, whether the cell is dead. Peripheral blood mononuclear cells (PBMCs) were separated and washed with OptiPrep Lymphocyte Separation Medium (AXIS-SHIELD) from the blood, and then suspended in RPMI 1640 cell culture medium (Gibco), and the concentration was adjusted to 10 6 / ml, add 1-2 drops of trypan blue staining solution (The British DrugHouses.LTD), and count under the microscope after 5 minutes. Live cells are transparent, and dead cells become blue opaque cells due to membrane damage, and the dye enters the cytoplasm. The number of blue cells in 100 cells was counted, that is, the percentage of cell death.
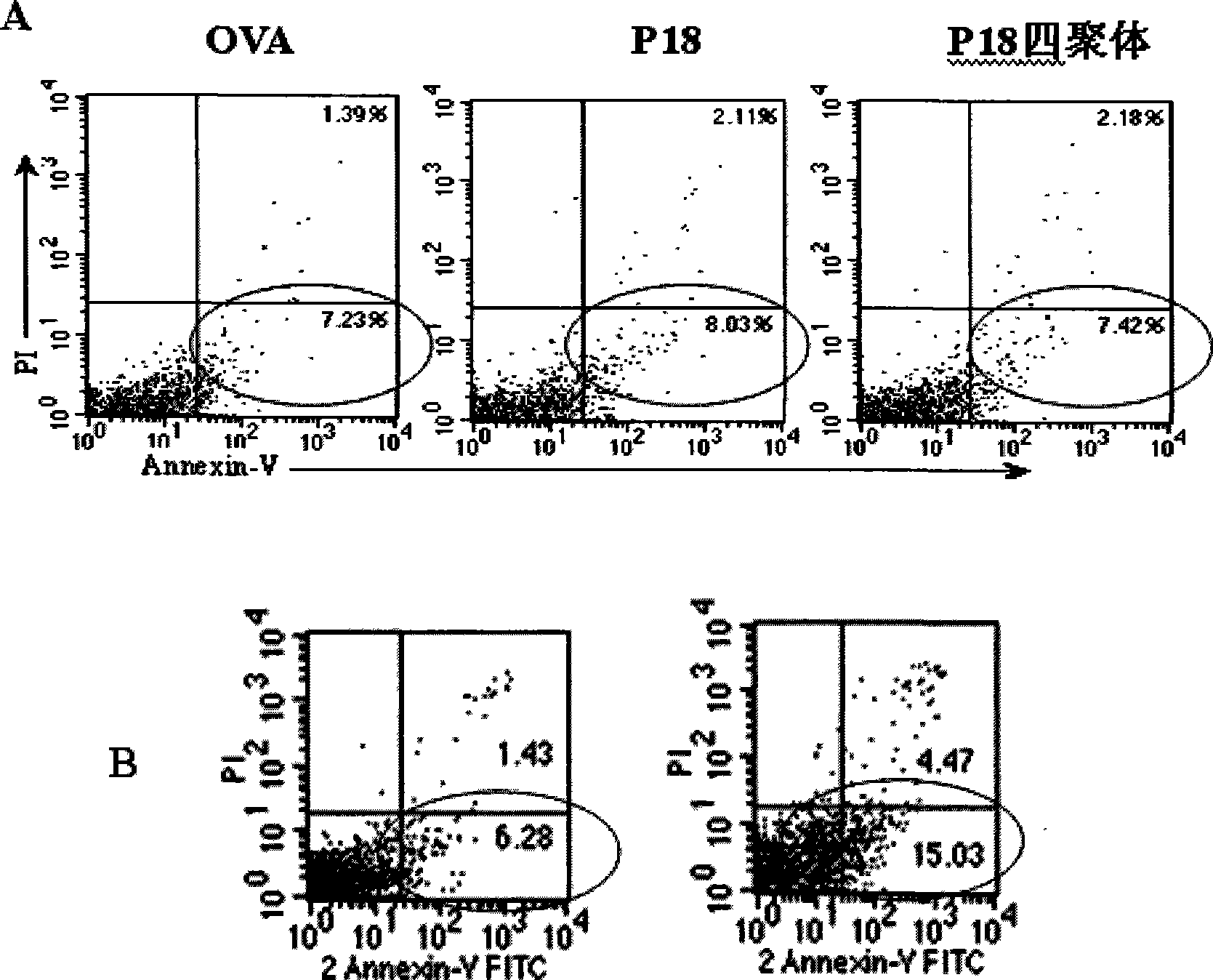
[0051] (2) Apoptosis was detected by Annexin V / PI double staining.
[0052] Annexin-V / PI apoptosis kit (BD PharMingen) was used.
[0053] The exper...
Embodiment 3
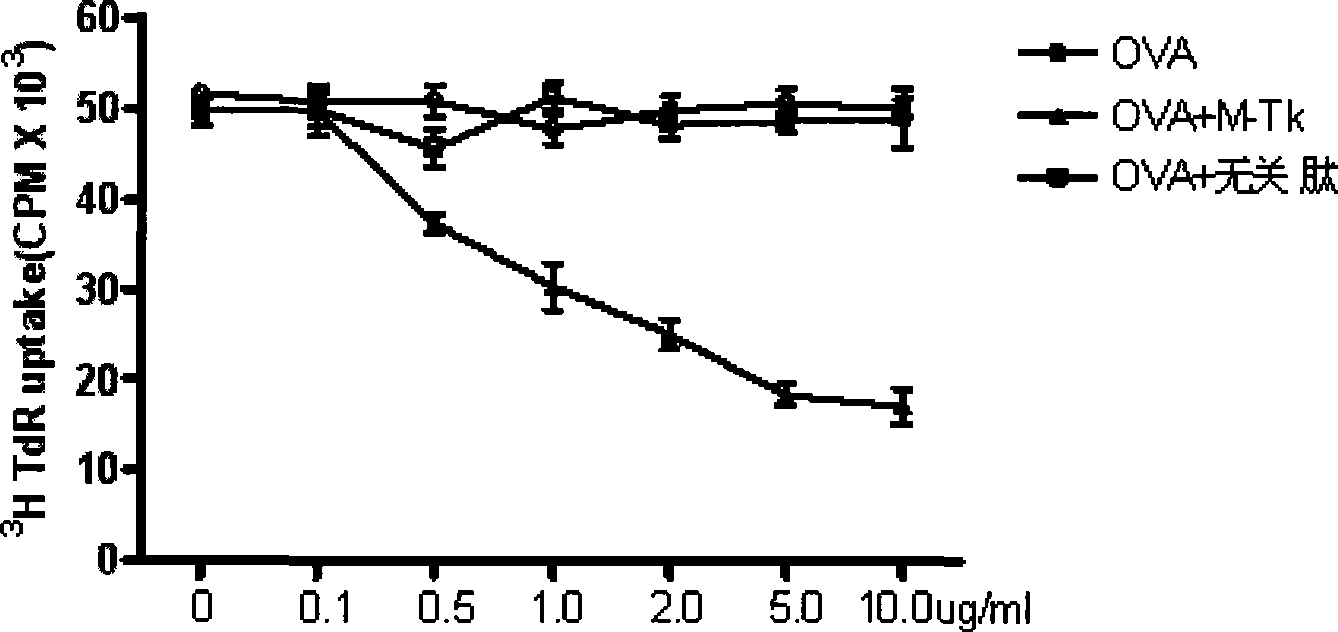
[0061] Comparing the biological activities of Trichosanthes whole protein and related derived peptides in vitro and in vivo
[0062] (1) Materials
[0063] Ovalbumin OVA, Complete Freund's Adjuvant CFA (Sigma); Fetal Bovine Serum (Hyclone Lab, Inc), RPMI1640 Medium (Invitrogen Corp); 3 H-TdR (Shanghai Institute of Nuclear Research); crystalline pure trichosanthin (Shanghai Jinshan Pharmaceutical Factory), trichosanthin-derived peptide p18 tetramer and P18 peptide (prepared in Example 1), MOG 35-55 (MEVGWYRSPFSRVVHLYRNGK) (synthesized by Shanghai GL Biochem Company). The unrelated peptide is a tetrameric peptide with the same structure as the p18 tetramer, and its relative molecular weight is 13.7kDa, which is equivalent to that of the p18 tetramer. The following reagents were also used in the experiment: OptiPrep Lymphocyte Separation Solution (AXIS-SHIELD); Mouse CD4, CD8 Magnetic Bead Separation Reagent (Miltenyi Biotech); PE-Cy5-CD3, FITC-CD8, PE-CD28, APC-CTLA4 flow For...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com