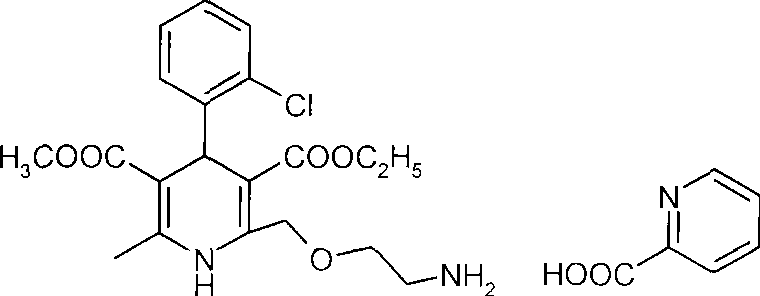
Therapeutic composition containing amlodipine salt and losartan medicine
A composition and technology of dipine salts, which are applied in the field of amlodipine series salts, can solve the problem of slow onset of amlodipine, affecting the bioavailability and onset time of amlodipine, and the lack of light resistance of amlodipine besylate, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
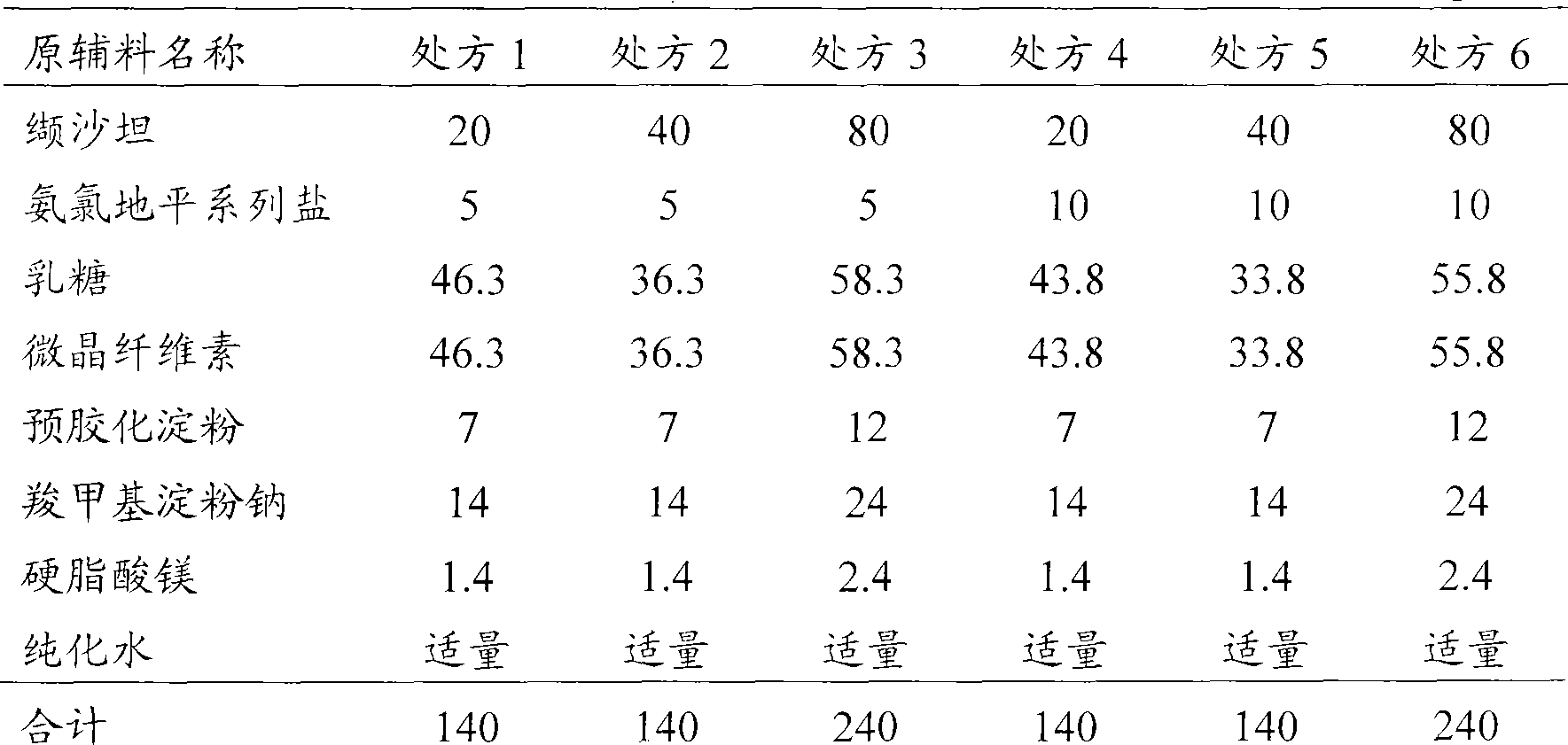
[0074] Embodiment 1 The general preparation method of valsartan / amlodipine series salt tablet
[0075] Valsartan and amlodipine series salts were pulverized, passed through a 100-mesh sieve, and set aside; lactose, microcrystalline cellulose, pregelatinized starch, carboxymethyl starch sodium, and magnesium stearate were respectively passed through a 80-mesh sieve, set aside.
[0076] Take amlodipine series salts and valsartan according to the prescription quantity, mix uniformly, and then mix homogeneously with carboxymethyl starch sodium, lactose, microcrystalline cellulose and pregelatinized starch successively according to the equal amount incremental method.
[0077] Add an appropriate amount of purified water to make a uniform soft material, pass through a 20-mesh sieve to granulate, dry at 50°C for 2 hours, add the prescribed amount of magnesium stearate, pass through a 20-mesh sieve for granulation, and mix well.
[0078] Determine the particle content, and adjust the ...
Embodiment 2
[0081] Embodiment 2 Light fastness experiment
[0082] The pharmaceutical composition of the research product amlodipine series salts (5 mg) and valsartan (80 mg) is as described in Examples 1-4 (specifically the following table). The research article and the reference article (amlodipine besylate / valsartan composition) were exposed to 50°C and placed under an incandescent lamp (220V, 100W) at 30cm above the sample for 4 weeks, and then the relative composition of each composition was determined. substance. It is evident from the above results that, compared with the reference product, the study product exhibited improved photostability except for the L-aspartate amlodipine / valsartan and maleate amlodipine / valsartan compositions Properties (in the table below, according to the stability of the related substances and compositions produced, they are arranged from high stability to low stability).
[0083] Table 2. Photostability of the composition of amlodipine series salts
...
Embodiment 3
[0086] Embodiment 3 Amlodipine series salt solubility test
[0087] Sufficient solubility is a necessary condition for the drug to obtain good bioavailability. However, amlodipine besylate does not have sufficient solubility in water, especially when it is close to the physiological pH7.4. Therefore, we also investigated camphorsulfonic acid Solubility of amlodipine, amlodipine pyroglutamate, amlodipine L-aspartate, amlodipine maleate, amlodipine mesylate and amlodipine besylate (as reference substance) As we all know, the pH of the above-mentioned saturated salt was also investigated, and the closer to pH 7.4, the better the biocompatibility. From the above results, it is evident that the study articles all exhibited improved solubility compared to the control, while having better or comparable pH at saturation, compared to the control.
[0088] Table 4. Solubility and saturation pH of amlodipine series salts
[0089]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com