Sustained-release composition and method for preparing the same
A technology of sustained release and composition, which is applied in drug delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as increased treatment costs and inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
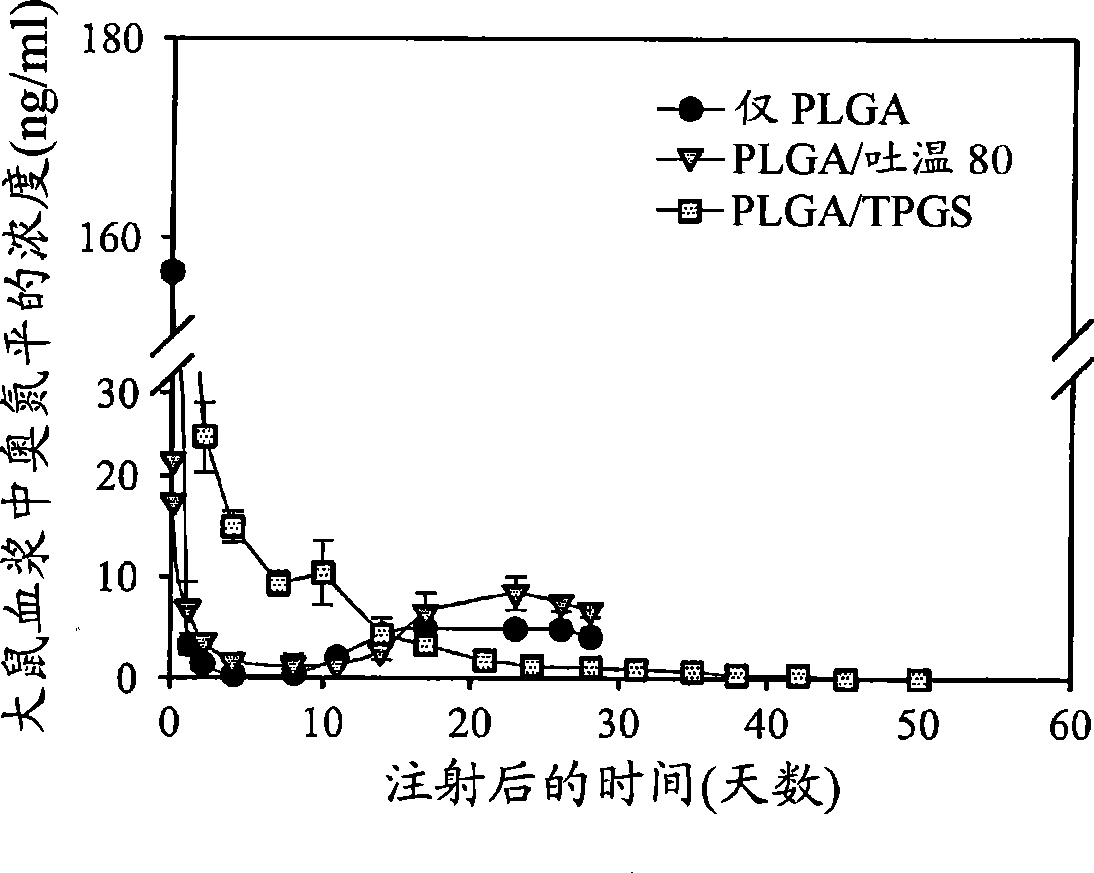
Image
Examples
Embodiment 1
[0032] Example 1. 50 / 50 PLGA with 0, 7, 26 and 42% Vitamin-E TPGS
[0033] Mix 80 mg of olanzapine, 200 mg of PLGA (LA / GA (lactic acid / glycolic acid) ratio = 50 / 50, M.W. = 43000) and different amounts of vitamin E-TPGS (accounting for olanzapine, PLGA and vitamin 0, 7, 26, 42% by weight of the total weight of E-TPGS) were dissolved together in 5 mL of dichloromethane to form an oil phase. The oil phase was added dropwise into 1000 mL of ice-water phase, the water phase contained 0.1% polyvinyl alcohol (PVA); and emulsified at 1000 rpm. The obtained oil / water (o / w) emulsion was continuously stirred at room temperature for 3 hours, the microspheres were collected by centrifugation, washed with F68 and water, and freeze-dried. The particle size and drug coating rate of the microspheres were analyzed by Multisizer and high performance liquid chromatography (HPLC), respectively. The results showed that the particle diameters of microspheres containing 0, 7, 26 and 42% (weight / wei...
Embodiment 2
[0034] Example 2. 85 / 15 PLGA Microspheres Containing 0, 4, 15 and 26% Vitamin E-TPGS
[0035] 320mg of olanzapine, 800mg of PLGA (LA / GA ratio=85 / 15, M.W.=53000) and different amounts of vitamin E-TPGS (accounting for 0, 4, 15, 26% by weight) were dissolved together in 15 mL of dichloromethane to form an oil phase. The oil phase was added dropwise into 2000 mL of ice-water phase, wherein the water phase contained 0.1% polyvinyl alcohol (PVA), and emulsified at 1000 rpm. The stirring, collection and analysis methods of this embodiment are the same as those of Example 1. The results showed that the particle diameters of microspheres containing 0, 4, 15 and 26% (weight / weight) vitamin E-TPGS were 40.0 ± 21.3, 58.8 ± 28.4, 47.4 ± 21.4 and 62.9 ± 25.9 μm, and the drug coating rate They are 77.2, 74.7, 87.6 and 83.7% respectively.
Embodiment 3
[0036] Example 3. 50 / 50 PLGA Microspheres Containing Different Aqueous Phases
[0037] 80mg of olanzapine, 200mg of PLGA (LA / GA ratio=50 / 50, M.W.=43000) and 42% (w / w) vitamin E-TPGS (to account for olanzapine, PLGA and vitamin E-TPGS total % by weight) were dissolved together in 5 mL of dichloromethane to form an oil phase. The oil phase was added dropwise into 1000 mL of ice-water phase, and emulsified at 1000 rpm; wherein the ice-water phase contained 0.05% PVA, or 0.05% PVA containing 0.5% gelatin. The obtained oil / water emulsion was continuously stirred at room temperature for 3 hours, and the microspheres were collected by centrifugation, washed with F68 and water, and freeze-dried. The analysis methods of particle size and drug coverage rate in this embodiment are the same as above. The results showed that the particle diameters of microspheres without gelatin and 0.5% gelatin were 108.7±37.3 and 85.5±31.4 μm, respectively, and their drug coating ratios were 74.1 and 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com