Medicine novel use of procyanidine oligomer and multimer
A technology with low proanthocyanidins and proanthocyanidins, applied in the field of medicine, can solve the problems of difficult complete excision, strong invasion and metastasis ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1, using human glioma U-87 and U251 cell lines, human leukemia HL-60 cell lines, human liver cancer Hep3B cell lines, human colon cancer Colon-205 cell lines, human Prostate cancer Du-145 cell line and human A549 lung cancer cell line were used as target cells to carry out the inhibitory effect experiment of F2 and F3 on these cells
[0023] 1.1 Materials
[0024] Human malignant glioma cells U-87 and U251, human leukemia HL-60 cell line, human liver cancer Hep3B cell line, human colon cancer Colon-205 cell line, human prostate cancer Du-145 cell line Strains and human-derived A549 lung cancer cell lines: provided by ATCC. DMEM dry powder: available from Invitrogen / Gibco. Fetal bovine serum: purchased from TBD Company. Pancreatin: purchased from Beijing Huamei Biotechnology Co., Ltd. Tetramethylazothiazolium blue (MTT): purchased from Beijing Huamei Shengke Biotechnology Co., Ltd. F2 and F3 were provided by Professor Sun Baoshan of the Portuguese National R...
Embodiment 2
[0033] Example 2. Using the human glioma U-87 cell line as the target cell, the effect experiment of F2 and F3 on the chemotaxis of U-87 cells induced by formyl peptide
[0034] 2.1 Materials
[0035] 48-well chemotaxis plate: purchased from Neuro Probe Company. Polycarbonate membrane: purchased from Neuro Probe Company. Type I rat tail collagen: purchased from Gene Company. Formyl peptide (fMLF): purchased from Sigma. F2 and F3 were provided by Professor Sun Baoshan of the Portuguese National Resource Laboratory.
[0036] 2.2 Method
[0037] The detection of cell chemotaxis adopts the chemotaxis chamber method. The upper hole of the chemotaxis chamber is the U-87 cell suspension pre-incubated with non-cytotoxic doses of F2 and F3 for 1 h, and the lower hole is 10nM formyl peptide. A negative control group (the lower hole is blank buffer solution, the upper hole is blank cell suspension) and a positive control group (the lower hole is 10 nM formyl peptide, and the upper h...
Embodiment 3
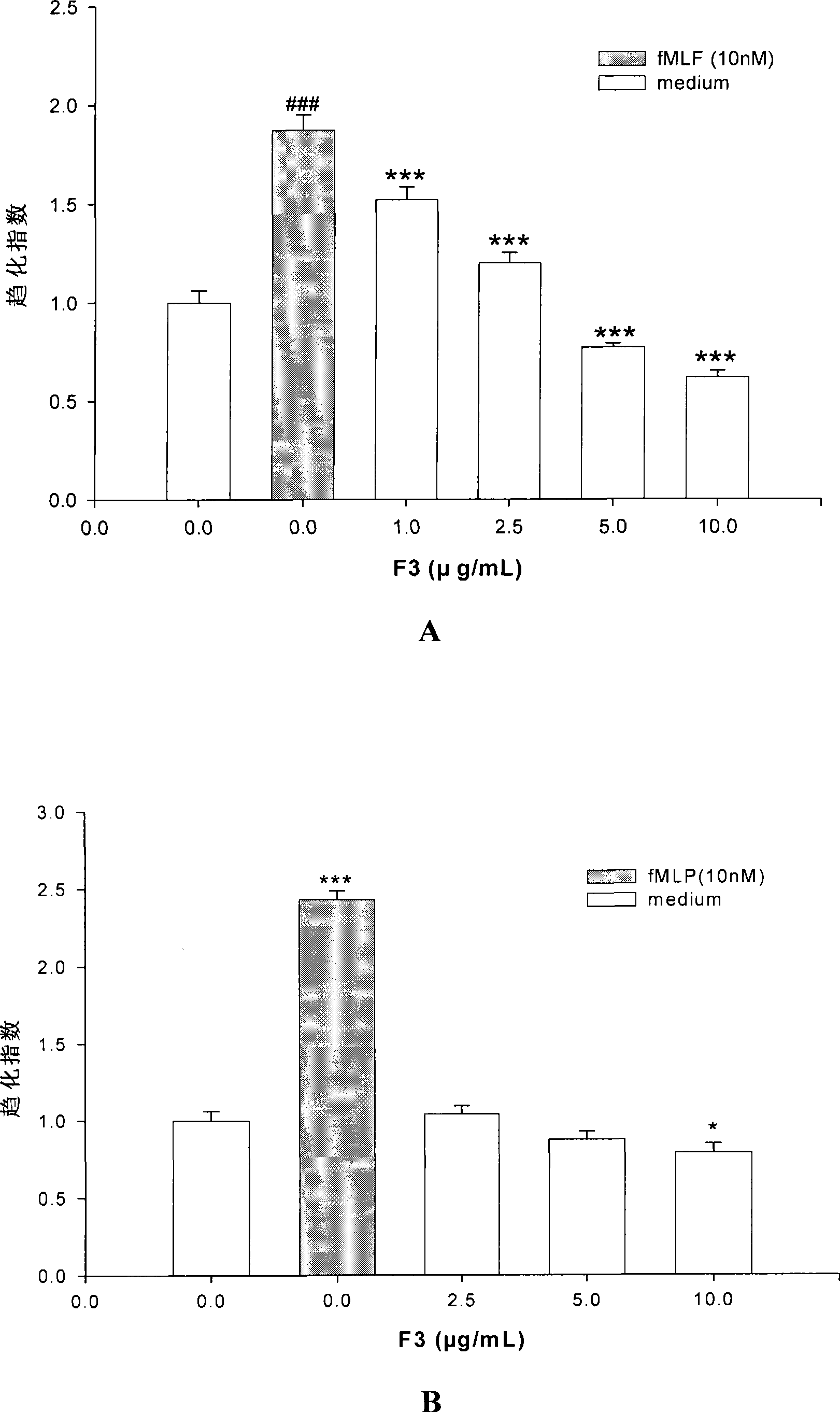
[0039] The result is as figure 1 As shown, F2 (2.5 μg / mL, 5 μg / mL and 10 μg / mL) pre-treated U87 cells for 1 h can significantly inhibit fMLF-induced chemotaxis of U-87 cells in a dose-dependent manner. Because F2 (2.5 μg / mL, 5 μg / mL) pre-treated U-87 cells for 1 h did not affect the random migration of U87, the inhibitory effect of F2 (2.5 μg / mL, 5 μg / mL) on fMLP-induced chemotaxis of U87 cells Does not work by affecting its random migration. Similarly, F3 (2.5 μg / mL, 5 μg / mL and 10 μg / mL) pre-treated U-87 cells for 1 h could significantly inhibit fMLF-induced chemotaxis of U-87 cells in a dose-dependent manner. Because F3 (2.5 μg / mL, 5 μg / mL) pre-treated U-87 cells for 1 h did not affect the random migration of U-87, so the F3 (2.5 μg / mL, 5 μg / mL) on the fMLF-induced U-87 cell Inhibition of Chlamylation does not work by affecting its random migration. Embodiment 3, the comparative experiment of the anti-glioma activity of total proanthocyanidins and F2, F3
[0040] 3.1 Ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com