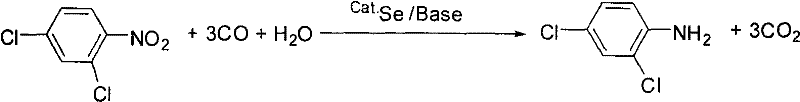A kind of method for synthesizing 2,4-dichloroaniline
A technology of dichloroaniline and dichloronitrobenzene, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., can solve problems such as serious environmental pollution, achieve high reduction selectivity, and facilitate large-scale The effect of industrial production and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 19.2 g (0.1 mol) of 2,4-dichloronitrobenzene, 0.32 g (4 mmol) of Se, 20 mL (1.1 mol) of water, 4 g (50 mmol) of sodium acetate and 0.2 L of toluene were added to a 1000 mL autoclave. After closing the autoclave and checking the airtightness, nitrogen gas was introduced to a pressure of 2 MPa, and then released. Replace like this 3 times, to remove the air in the autoclave, feed carbon monoxide to a pressure of 2Mpa, then deflate, and then feed carbon monoxide to a pressure of 3Mpa. Heat to 140°C to react for 4 hours, cool to room temperature, release the waste gas after reaction to normal pressure, stir in the air for 2 hours, filter out the catalyst, and distill the filtrate under reduced pressure to obtain the product 2,4-dichloroaniline 16.1 g (0.1 mol), yield 99%.
Embodiment 2
[0023] Reaction temperature, water yield, reaction time are as follows, and experimental method and other conditions are with embodiment 1, and yield is as follows:
[0024] Water volume (mol)
[0025] It can be seen from the table that the yield decreases as the amount of water increases, and the molar ratio of 2,4-dichloronitrobenzene to water is preferably 1:1 to 40.
Embodiment 3
[0027] The consumption of Se is as follows, and experimental method and other conditions are with embodiment 1, and yield is as follows:
[0028] Se content (mmol)
[0029] It can be seen from the table that with the increase of the amount of Se, the yield does not increase much. In consideration of cost reduction, the amount of Se is preferably 1% to 10% of that of 2,4-dichloronitrobenzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com