Novel coupling compound of bile acid and anti-hepatitis virus medicament and medical use thereof
An anti-hepatitis virus and bile acid technology, applied in the preparation of viral hepatitis therapeutic drugs, in the field of salt preparation, can solve the problems of complex chemical composition, small drug loading and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
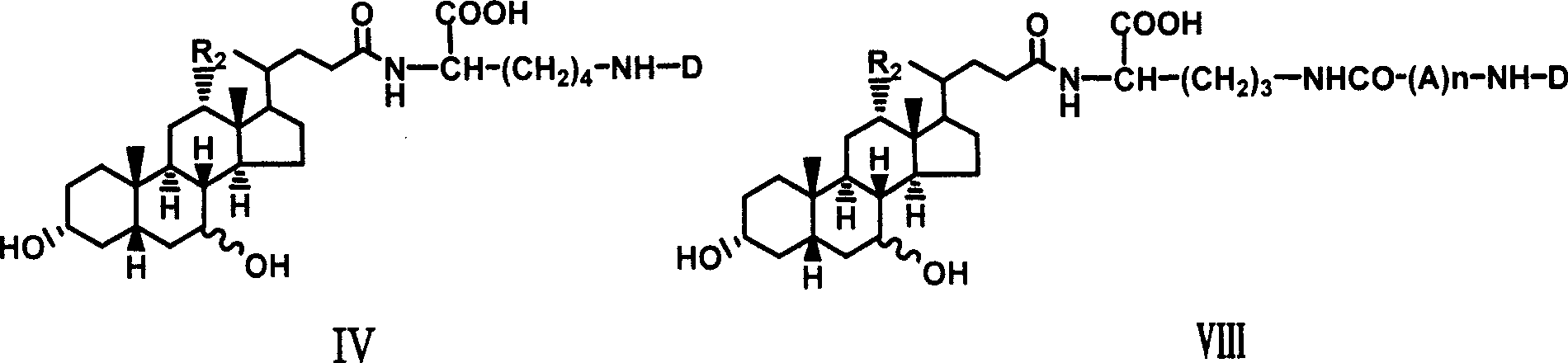
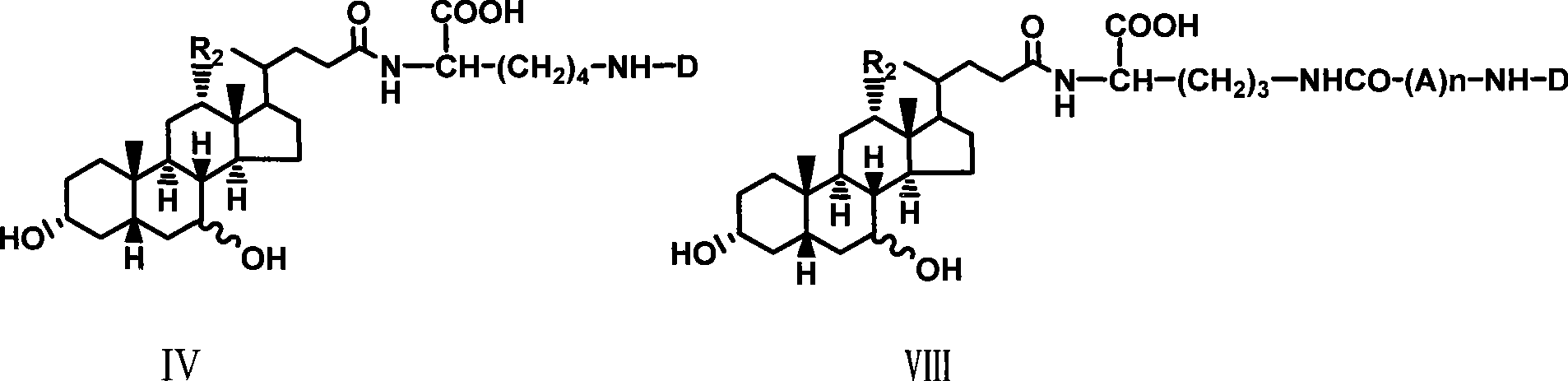
[0027] Example 1 Synthesis of α-cholyl-benzyloxycarbonyl-lysine methyl ester (I)
[0028] Weigh 1.08g (5.25mM) DCC and dissolve it in 5ml tetrahydrofuran, and put it in the refrigerator to cool. Weigh 2.04g (5mM) cholic acid and dissolve it in 10ml tetrahydrofuran, stir to dissolve it, cool in an ice-salt bath, add 0.766g (5mM) HOBt below 0°C, 2.4g ε-benzyloxycarbonyl-lysine methyl ester ( 5mM), 0.55ml N-methylmorpholine, stirred, kept below 0°C and poured into cold DCC solution in tetrahydrofuran, kept below 0°C and stirred for 2 hours, removed the ice bath, and stirred overnight at room temperature. The white insoluble solid was filtered off, and tetrahydrofuran was distilled off under reduced pressure to obtain a yellow viscous liquid, which was dissolved in ethyl acetate, washed successively with saturated NaHCO3 aqueous solution, 10% citric acid solution, saturated NaHCO3 aqueous solution and saturated NaCl aqueous solution, and the organic layer was collected and washed ...
Embodiment 2
[0030] Example 2 Synthesis of α-choyl-lysine methyl ester (II)
[0031] Weigh an appropriate amount of 10% palladium carbon in a round bottom bottle, add a small amount of anhydrous methanol to make it suspended under an ice bath (to prevent burning), dissolve 2 g of I with a small amount of methanol, and slowly pour it into the methanol solution of palladium carbon. Catalyzed hydrogenation until the reaction was complete, filtered off palladium carbon, washed with anhydrous methanol, combined the filtrates, evaporated the solvent under reduced pressure to obtain a light yellow viscous liquid, added anhydrous diethyl ether to grind it to solidify, and filtered to obtain 1.45 g of a near-white solid. Yield 90%.
[0032] TLC: developing solvent: ethyl acetate: methanol: ammonia water (6:4:1 drops), Rf=0.4
Embodiment 3
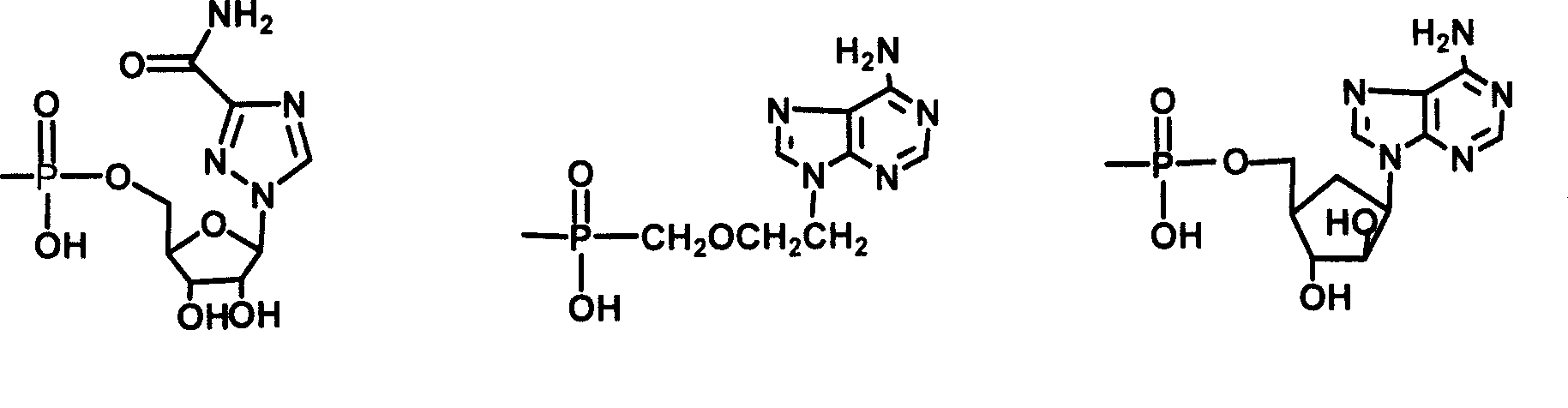
[0033] Example 3 Synthesis of choyl-lysine methyl ester-adenosine monophosphate (III)
[0034]Dissolve 0.5g II in 10ml Na2CO3 / NaHCO3 buffer solution of pH 9.50, add 1g adenosine adenosine monophosphate phosphoryl imidazole (ara-AMPIm), react at 40°C for 48 hours, remove the solvent under reduced pressure to obtain a viscous liquid , adding anhydrous ether, ground and solidified, and filtered to obtain a light yellow solid mixture. This mixture was separated with a 27×33cm thin-layer silica gel preparation plate, and 200 mg of the sample was loaded each time, dissolved in methanol, and evenly coated on the bottom of the thin plate, and washed with isopropyl Alcohol:methanol:ammonia water (6:3:1) was developed as a developer, and the product band at Rf0.52 was scraped off and eluted with methanol to obtain 0.3g of light yellow solid (III), with a yield of 40%.
[0035] TLC: developing solvent: isopropanol: methanol: ammoniacal liquor (6:3:1), Rf=0.45
[0036] MS(m / e):894.9(M+1)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com