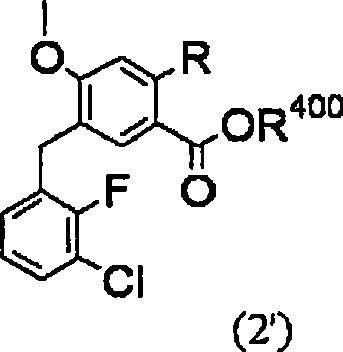
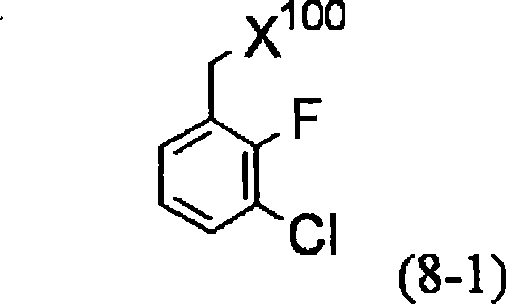
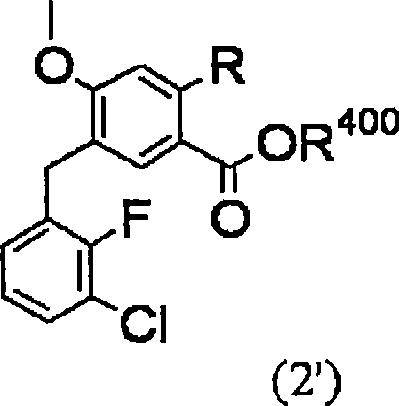
Method for producing 4-oxoquinoline compound
A compound and representative technology, applied in the field of preparation of anti-HIV drugs, can solve problems such as not providing compound descriptions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1095] One example of the production method of the present invention is explained below. However, the present invention is not limited thereto.
[1096] 【0443】
[1097] Even if there is no description of the preparation method, those of ordinary skill in the art can understand that effective preparation can be carried out by introducing protective groups into functional groups, removing protective groups during post-treatment, converting to desired functional groups at any stage, etc. as appropriate.
[1098] 【0444】
[1099] Post-treatment after the reaction of each step can be applied by a typical method in which isolation and purification are performed by selecting as appropriate or combining various conventional methods such as crystallization, recrystallization, distillation, partition, silica gel column chromatography, preparative HPLC, and the like.
[1100] In the following preparation methods and this specification, unless otherwise specified, "room temperature" gene...
Embodiment 1
[1682] step 1
[1683] Synthesis of 5-bromo-2,4-dimethoxybenzoic acid
[1684] 【0619】
[1685]
[1686] 【0620】
[1687] 2,4-Dimethoxybenzoic acid (30.0 g) was suspended in acetic acid (180 ml). A bromine (27.6 g) / acetic acid (60 ml) solution was slowly added dropwise to the suspension. After the dropwise addition, the mixture was stirred at 25° C. for 2 hours, and the reaction was confirmed to be terminated by HPLC. An aqueous solution of sodium sulfite (2.10 g) and water (360 ml) was added dropwise to the reaction mixture. After the dropwise addition was complete, the mixture was stirred at 25° C. for 1 hour. The precipitated crystals were filtered, washed with water (150ml) 4 times, and dried in vacuo to give 5-bromo-2,4-dimethoxybenzoic acid as white crystals (41.2g) (96%).
[1688] 【0621】
[1689] step 2
[1690] Synthesis of methyl 5-bromo-2,4-dimethoxybenzoate
[1691] 【0622】
[1692]
[1693] 【0623】
[1694] 5-Bromo-2,4-dimethoxybenzoic acid (10.0 g) and...
Embodiment 2
[1815] step 1
[1816] Synthesis of 3-chloro-2-fluorobenzylzinc bromide
[1817] 【0665】
[1818]
[1819] 【0666】
[1820] Under an argon atmosphere, suspend zinc powder (3.18g) in tetrahydrofuran (8ml), and add 1,2-dibromoethane (0.061g, 0.32mol) and trimethylsilyl chloride in sequence at 60°C (0.071 g, 0.65 mol), the mixture was stirred for 30 min. A solution of 3-chloro-2-fluorobenzyl bromide (7.48 g, 32.5 mol) in tetrahydrofuran (20 ml) was added dropwise to the above prepared solution at 60°C. With heating, the mixture was further stirred for 1 hour to obtain a solution of 3-chloro-2-fluorobenzylzinc bromide in tetrahydrofuran.
[1821] Step II
[1822] Synthesis of methyl 5-bromo-2-fluoro-4-methoxybenzoate
[1823] 【0667】
[1824]
[1825] 【0668】
[1826] With stirring under ice-cooling, concentrated sulfuric acid (14 ml) was added dropwise to methanol (840 ml). Then, 2-fluoro-4-methoxybenzoic acid (70.0 g) was added, and the mixture was stirred at 65° C. f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com