Synthetic oligopeptide and uses thereof
An oligopeptide and cysteinyl technology, which is applied in the agricultural field to achieve the effects of high biological activity, high physiological activity, improved stability and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
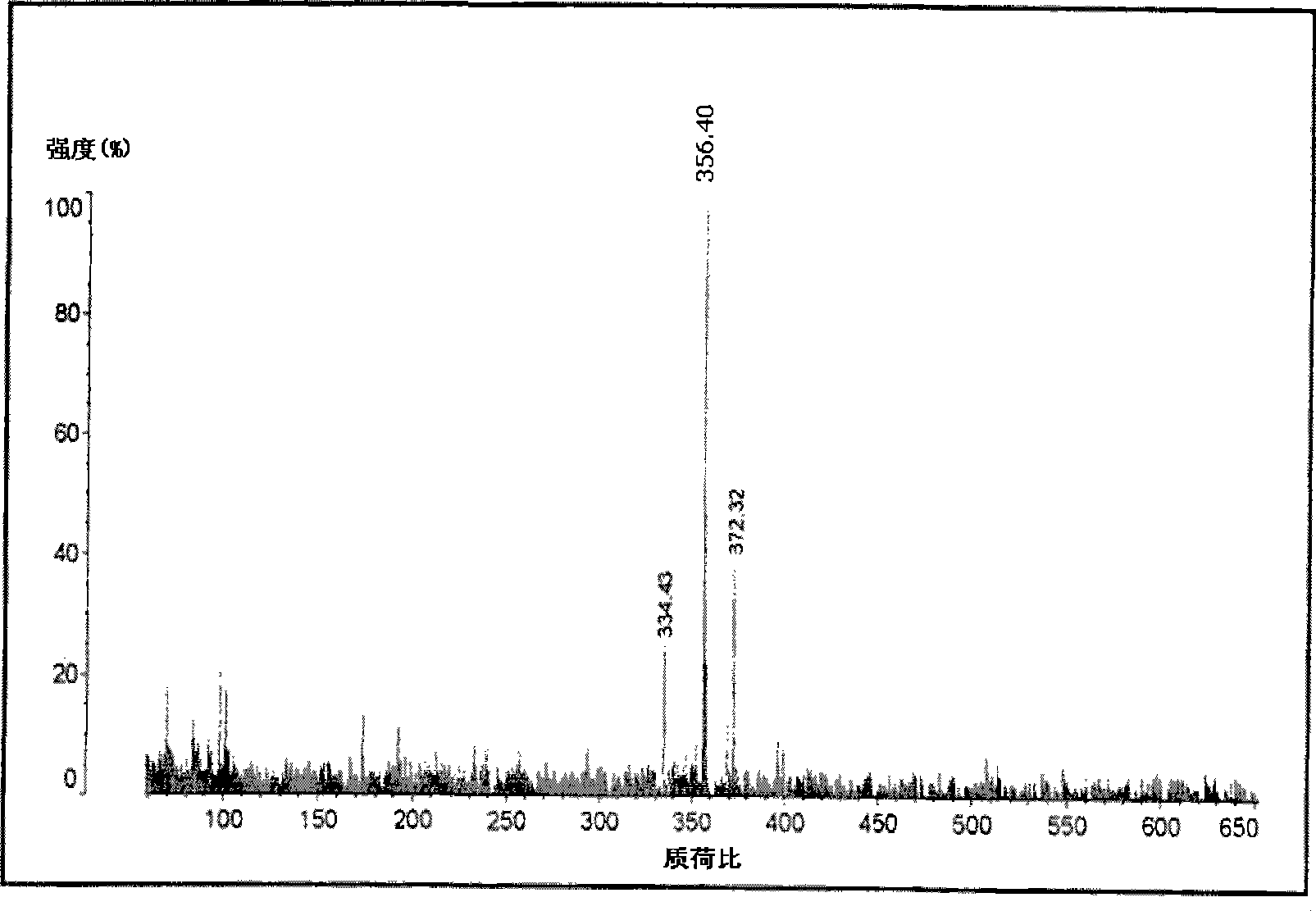
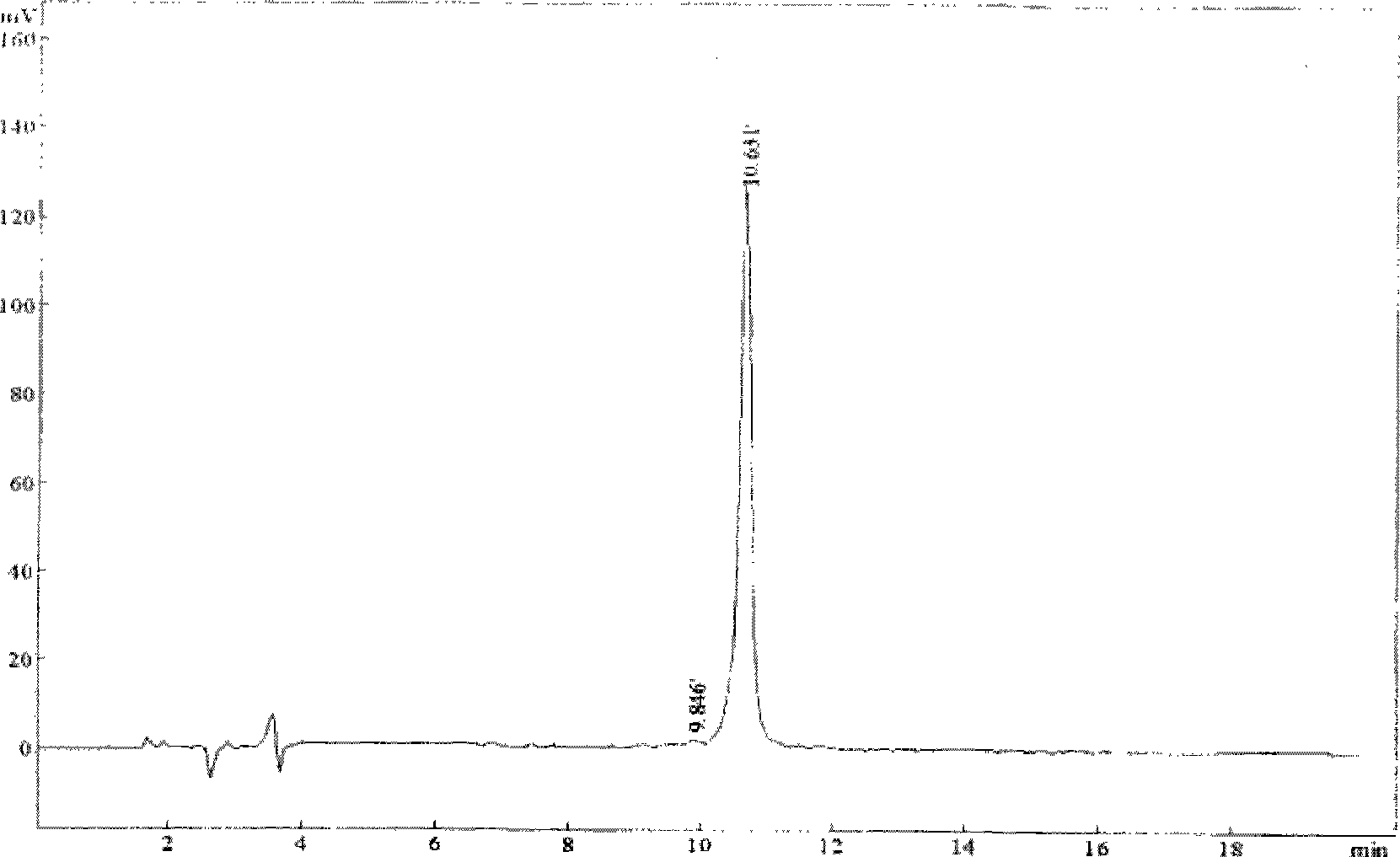
Image
Examples
Embodiment 1
[0018] Embodiment 1: Preparation of oligopeptide glutamyl-cysteinyl-γ-aminobutyric acid
[0019] Weigh 2 g of 2-chlorotrityl chloride resin, add 20 ml of N-methylpyrrolidone to swell the resin for 2 hours, and drain the liquid; add 20 ml of dichloromethane, mix for 1 minute, empty the reactor, and then add N - 20 ml of methylpyrrolidone, mix for 1 minute, empty the reactor, repeat 2 times; weigh 2 g of 9-fluorenylmethoxycarbonyl γ-aminobutyric acid, dissolve with 5 ml of N-methylpyrrolidone and transfer to the reactor , add 40 microliters of 3M diisopropylethylamine, mix well and add to the reactor, then add 15 milliliters of N-methylpyrrolidone to react for 2 hours. Empty the reactor, add 20 ml of dichloromethane, mix for 1 minute, empty the reactor, add 20 ml of N-methylpyrrolidone, mix for 1 minute, empty the reactor, repeat 2 times. Sampling for testing.
[0020] Add 20 ml of 20% hexahydropyridine, empty the reactor after 30 minutes of reaction, then add 20 ml of 20% hex...
Embodiment 2
[0026] Embodiment 2: the preparation of oligopeptide prolyl-cysteinyl-glycine (Pro-Cys-Gly)
[0027] Weigh 2 g of 2-chlorotrityl chloride resin, add 20 ml of N-methylpyrrolidone to swell the resin for 2 hours, and drain the liquid; add 20 ml of dichloromethane, mix for 1 minute, empty the reactor, and then add N -Methylpyrrolidone 20 ml, mix for 1 minute, empty the reactor, repeat 2 times; weigh 2 g of 9-fluorenylmethoxycarbonylglycine, dissolve and transfer to the reactor with 5 ml of N-methylpyrrolidone, add 40 μ liter of 3M diisopropylethylamine was added into the reactor after mixing, and then 15 ml of N-methylpyrrolidone was added to react for 2 hours. Empty the reactor, add 20 ml of dichloromethane, mix for 1 minute, empty the reactor, add 20 ml of N-methylpyrrolidone, mix for 1 minute, empty the reactor, repeat 2 times. Sampling for testing.
[0028]Add 20 ml of 20% hexahydropyridine, empty the reactor after 30 minutes of reaction, then add 20 ml of 20% hexahydropyrid...
Embodiment 3
[0033] Example 3: Synthetic oligopeptides scavenge free radicals under in vitro conditions
[0034] 1. Experimental method
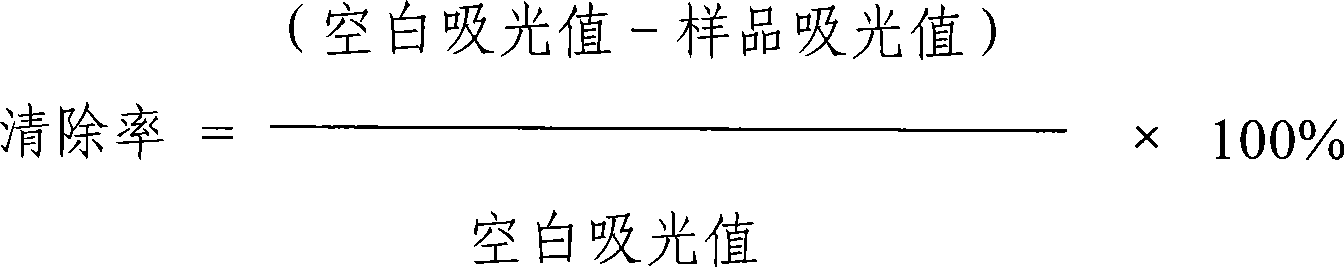
[0035] The scavenging of free radicals by oligopeptides under in vitro conditions is evaluated by the scavenging rate of 1,1-diphenylpicrylphenylhydrazine (DPPH) free radicals: the oligopeptides are configured into a 0.2mmol / L solution, and DPPH ethanol The solution was mixed and reacted for 30 minutes, and the absorbance value was measured at a wavelength of 517 nm with a CARY 100 Bio UV-visible spectrophotometer (CARY 100 Bio UV-visible spectrophotometer, produced by VARIAN, USA), with acetic acid buffer as a blank and GSH as a positive control. The in vitro clearance rate of oligopeptide to DPPH was calculated by the following formula:
[0036]
[0037] Each sample was repeated 5 times and the average value was taken.
[0038] 2. Experimental results
[0039] The in vitro scavenging rate of the eight synthetic oligopeptides on organic free radic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com