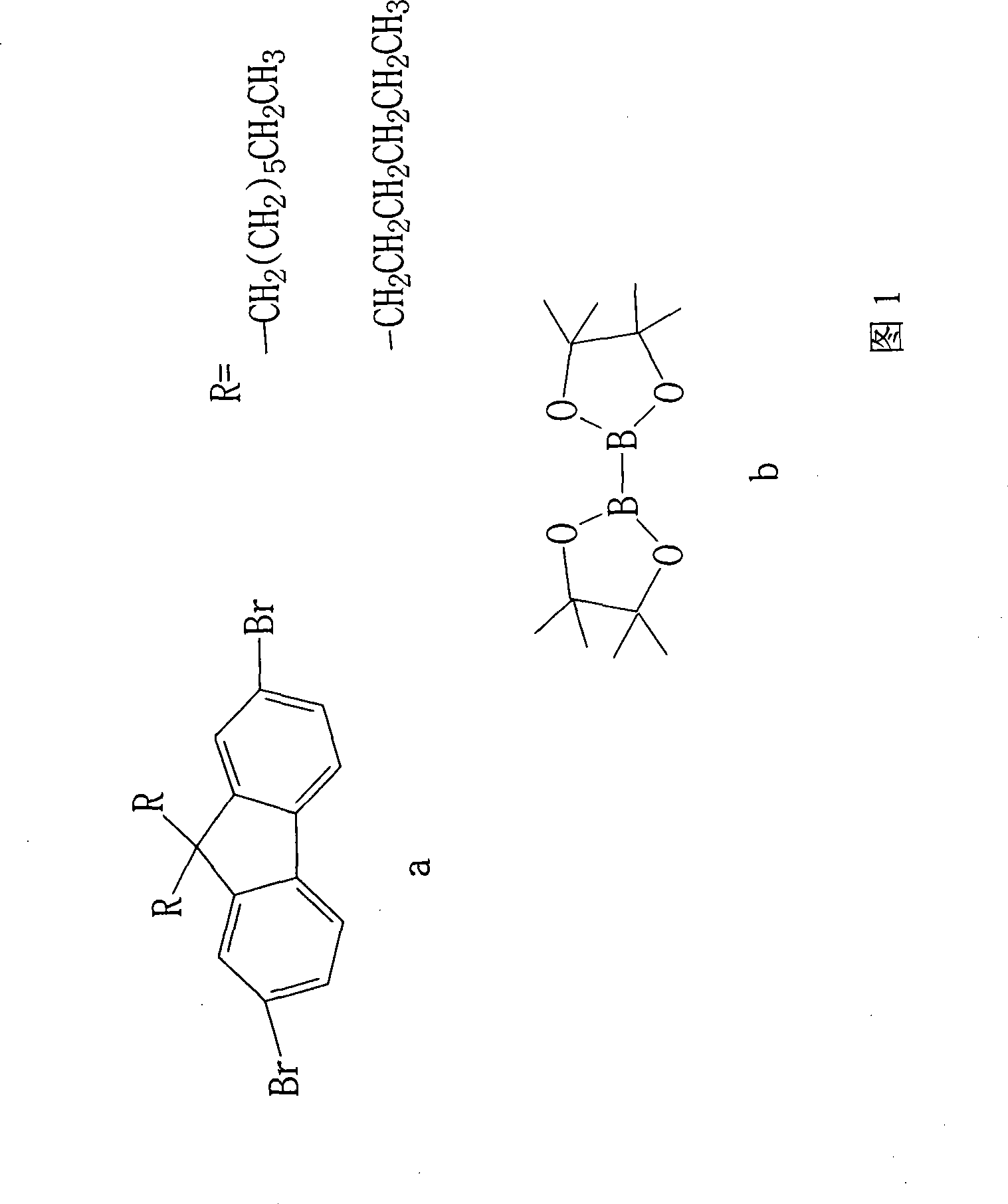
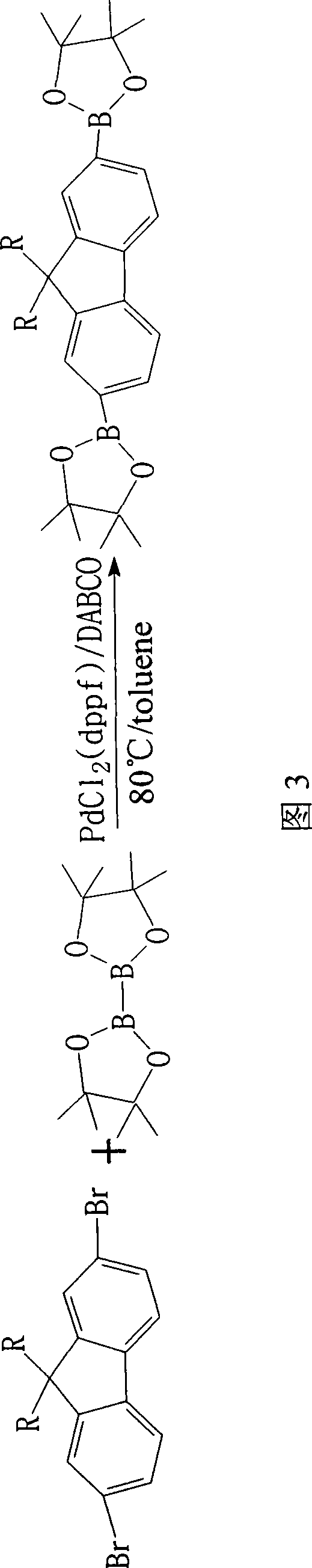Synthesis of esterifiable fluorene diborate
A synthesis method and the technology of dialkyl diboronic esters are applied in the field of new method synthesis of dialkyl diboronic esterified fluorene monomers, which can solve the problems of unsuccessful synthesis of diboronic esters, increased difficulty of experiments, and problems in experiments. Harsh conditions and other problems, to achieve the effect of great practical value, simple post-processing, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Reactant 2,7-dibromo-9,9-dioctylfluorene and catalyst PdCl 2 The molar ratio of (dppf) is: 1:6%
[0026] Catalyst PdCl 2 The molar ratio of (dppf) to DABCO is: 1:16
[0027] Add 2mmol 2,7-dibromo-9,9-dioctylfluorene, 4.4mmol double pinacol borate 12%mmolPdCl in a 100ml four-necked flask equipped with a thermometer and a magnetic stirrer 2 (dppf), 192% mmol DABCO, 12 mmol potassium acetate and 10 ml toluene, vacuumize and feed nitrogen, and continuously stir and react for 4 h under the condition of heating in an oil bath at 80°C. The organic layer was separated, the aqueous layer was extracted several times with toluene, the organic layers were combined, and the organic layer was washed several times with water, then dried with anhydrous magnesium sulfate, and then passed through a column (silica gel as the solid phase, toluene as the mobile phase), and the Evaporate the solvent to dryness with an evaporator to obtain a crude product as a pale yellow solid, and finall...
Embodiment 2
[0029] Reactant 2,7-dibromo-9,9-dioctylfluorene and catalyst PdCl 2 The molar ratio of (dppf) is: 1:6%
[0030] Catalyst PdCl 2 The molar ratio of (dppf) to DABCO is: 1:20
[0031] Add 2mmol 2,7-dibromo-9,9-dioctylfluorene, 4.4mmol double pinacol borate 12%mmolPdCl in a 100ml four-necked flask equipped with a thermometer and a magnetic stirrer 2 (dppf), 240% mmol DABCO, 12 mmol potassium acetate and 10 ml toluene were vacuumed and nitrogen gas was introduced, and the reaction was continuously stirred for 4 h under the condition of heating in an oil bath at 80°C. The organic layer was separated, the aqueous layer was extracted several times with toluene, the organic layers were combined, and the organic layer was washed several times with water, then dried with anhydrous magnesium sulfate, and then passed through a column (silica gel as the solid phase, toluene as the mobile phase), and the Evaporate the solvent to dryness with an evaporator to obtain a crude product as a pa...
Embodiment 3
[0033] Reactant 2,7-dibromo-9,9-dioctylfluorene and catalyst PdCl 2 The molar ratio of (dppf) is: 1:4%
[0034] Catalyst PdCl 2 The molar ratio of (dppf) to DABCO is: 1:4
[0035]Add 2mmol 2,7-dibromo-9,9-dioctylfluorene, 4.4mmol double pinacol borate 8%mmolPdCl to a 100ml four-necked flask equipped with a thermometer and a magnetic stirrer 2 (dppf), 32% mmol DABCO, 12 mmol potassium acetate and 10 ml toluene, vacuumize and blow in nitrogen, and continuously stir and react for 6 h under the condition of heating in an oil bath at 80°C. The organic layer was separated, the aqueous layer was extracted several times with toluene, the organic layers were combined, and the organic layer was washed several times with water, then dried with anhydrous magnesium sulfate, and then passed through a column (silica gel as the solid phase, toluene as the mobile phase), and the Evaporate the solvent to dryness with an evaporator to obtain a crude product as a pale yellow solid, and finally...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com