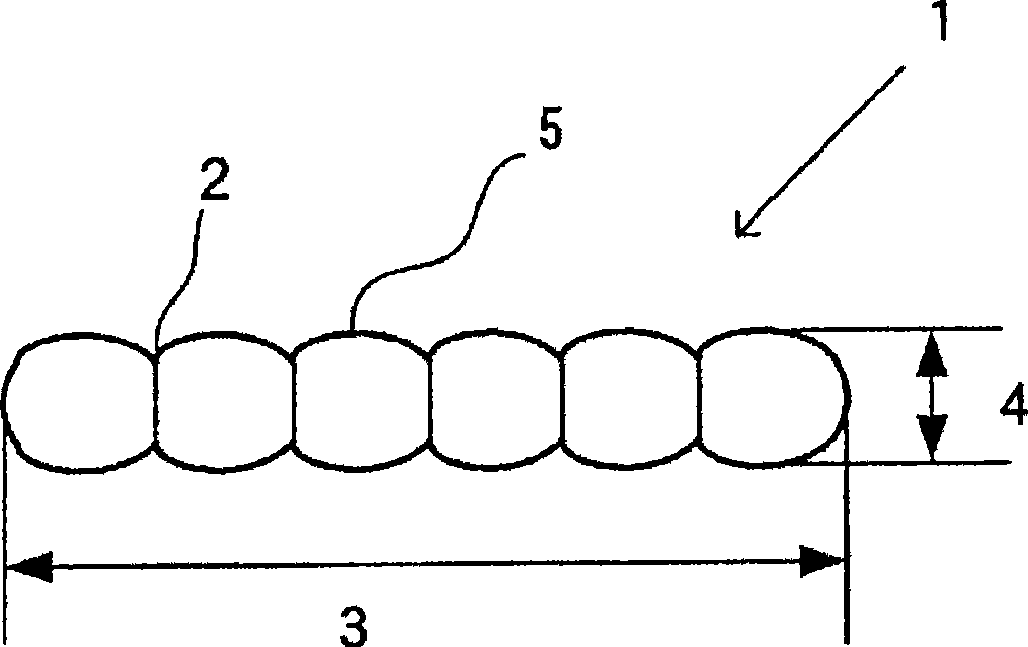
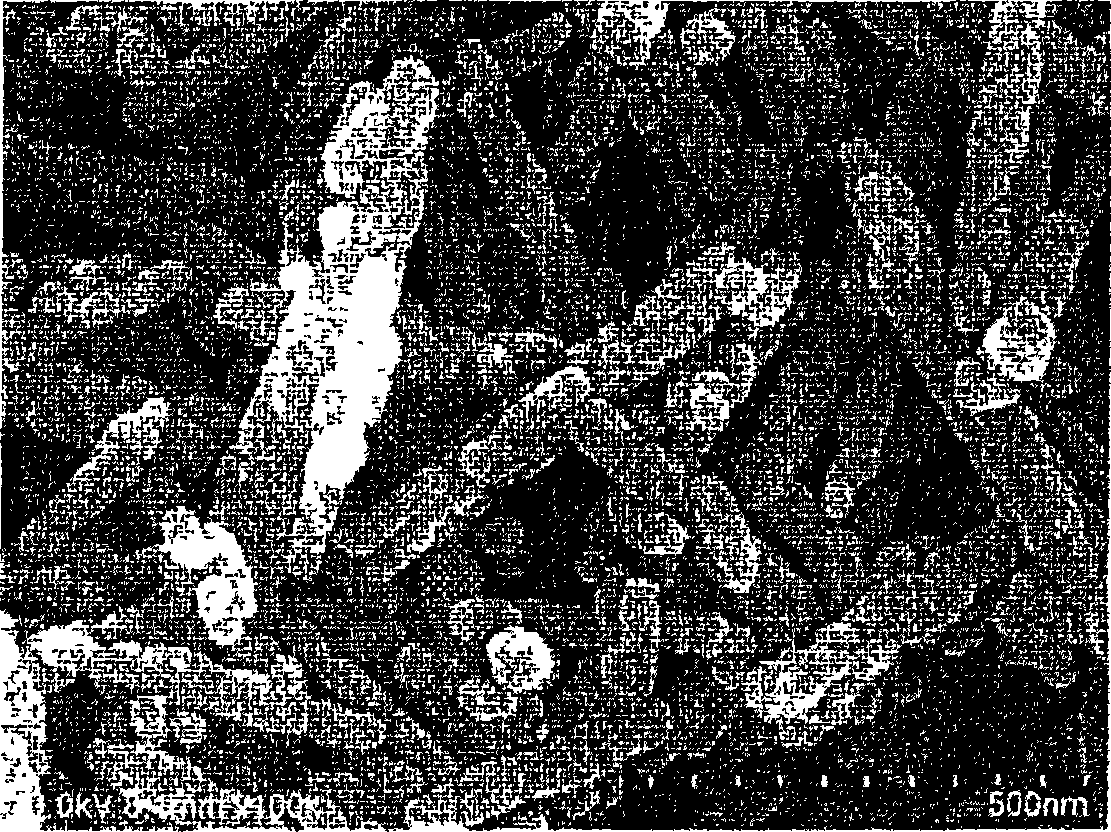Barium carbonate particle powder, production method thereof, and production method of perovskite barium titanate
A manufacturing method and technology of barium carbonate, applied in the direction of barium carbonate, calcium carbonate/strontium/barium, titanate, etc., can solve the problems of impurity mixing, purity, dispersibility, residue, etc., and achieve the suppression of impurity mixing and good dispersibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0119] 50.0 g of barium hydroxide octahydrate, 0.156 g of commercially available citric acid (100% in terms of purity), and 1,500 g of water were added to a reaction vessel to dissolve barium hydroxide and citric acid. Then, while stirring the reaction solution at 10,000 rpm, 0.0056 g of carbon dioxide was blown in at a flow rate of 0.0112 g / min to react, and needle-shaped barium carbonate was deposited. The reaction temperature at this time was 20° C., and the reaction time was 30 seconds.
[0120] Next, the 4% by weight slurry after the reaction was completed was put into an airtight container (autoclave), and heat-treated under stirring. The heat treatment temperature and time are shown in Table 1.
[0121] After heat treatment, the slurry was separated from solid and liquid by conventional methods, dried at 120° C. for 6 hours, and pulverized to obtain barium carbonate, which was used as a rod-shaped barium carbonate particle powder sample.
Embodiment 6~7
[0123] The reaction vessel is changed into an open vessel (flask) by a closed vessel (autoclave), and heat treatment is carried out according to the temperature and time shown in Table 1, except that, obtain rod-shaped barium carbonate under the same conditions as in Examples 1 to 5 Granular powder sample.
Embodiment 8
[0125] Precipitate needle-shaped barium carbonate in the same way as in Example 1, then in the process of heat treatment, the slurry is concentrated to 12% by weight and heat-treated, except that, the same operation as in Example 1 is performed to obtain rod-shaped barium carbonate particle powder sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com