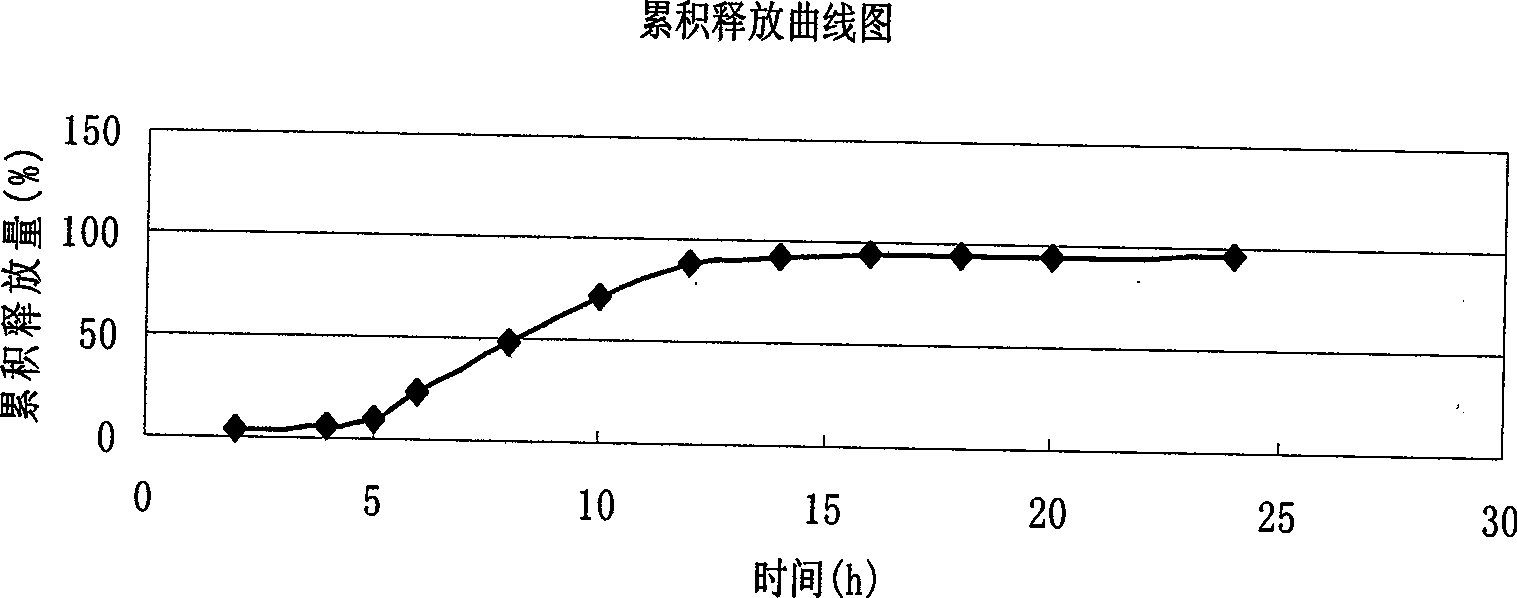
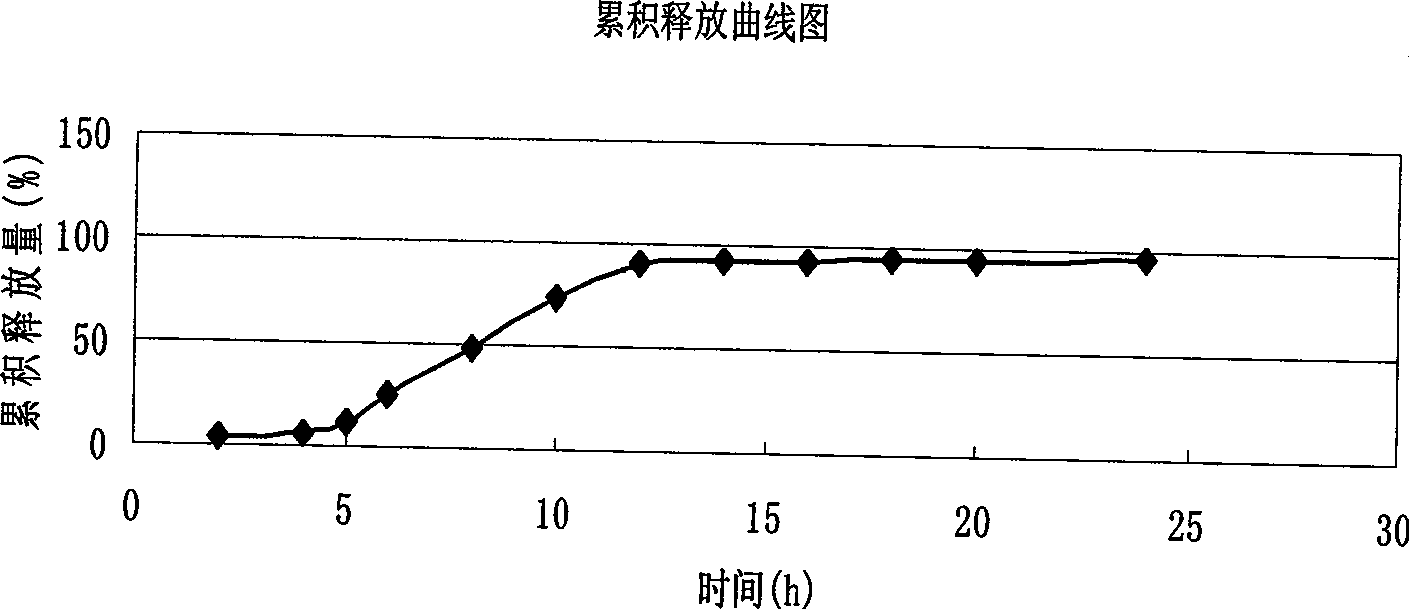
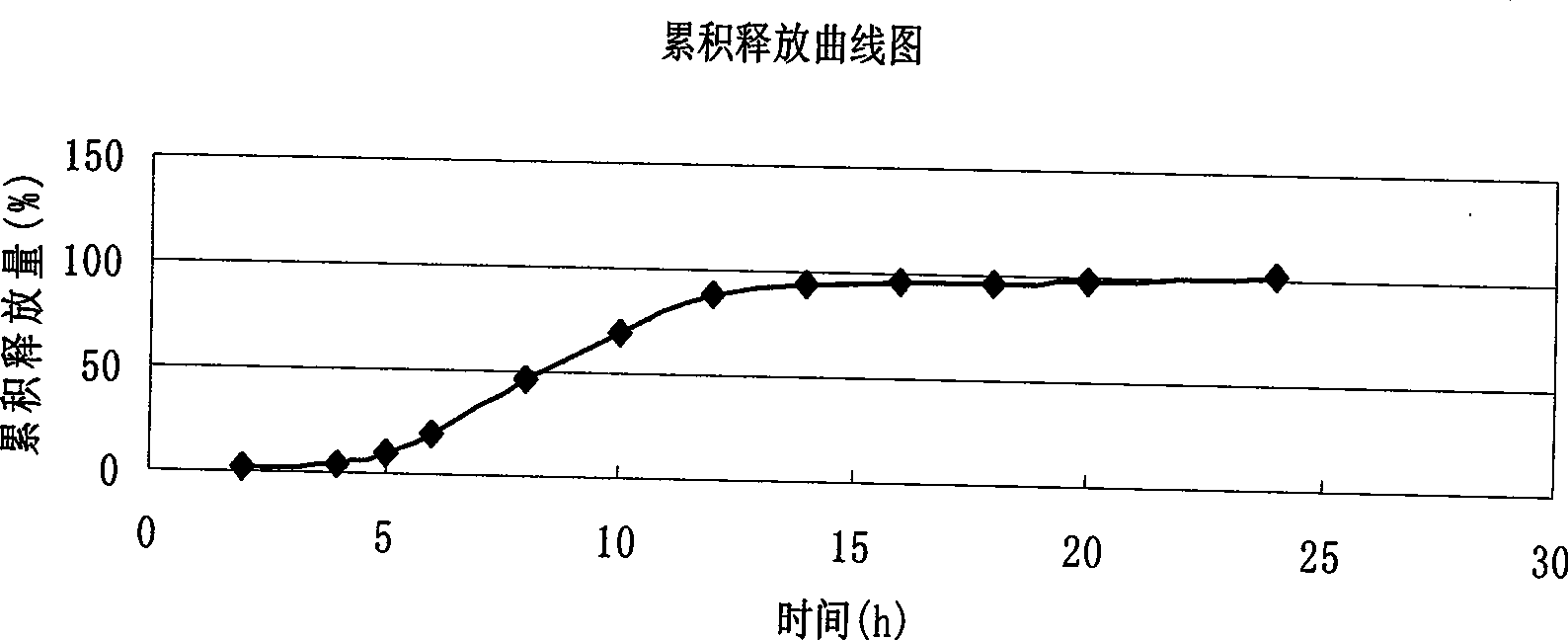
Verapamil hydrochloride delayed-release capsule and preparation method thereof
A technology of verapamil hydrochloride and capsules, which can be applied to cardiovascular system diseases, active ingredients of nitrile/isonitrile, active ingredients of heterocyclic compounds, etc., can solve the problem of short maintenance time of effective blood concentration, unfavorable industrialized large-scale production, and short biological half-life and other problems, to achieve the effect of good reproducibility of absorption kinetics, improved bioavailability, and small inter-individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation of verapamil hydrochloride containing pill core:
[0030] (1) Pass 120g of verapamil hydrochloride, 52g of microcrystalline cellulose and 2g of magnesium stearate through a 100-mesh sieve respectively, mix well, add 4g of purified water to make a suitable soft material, put the prepared soft material into the extruder Out of the extruded material tank of the spheronizer, the soft material is extruded and spheronized to obtain a wet pill core, which is put into a fluidized bed for fluidization drying to obtain a dry pill core for use;
[0031] Preparation of pellets:
[0032] (2) Take 42g hypromellose E5 and add it into purified water while stirring, ultrasonically dissolve and make the solution uniform, and make a solution with a concentration of 3% by weight as the swelling layer coating solution; another step ( 1) The prepared drug-containing pellet core is placed in a fluidized bed, preheated for 15 minutes at an inlet air temperature of 40° C., and ...
Embodiment 2
[0041]
[0042] Preparation method: preparation of verapamil hydrochloride containing pill core:
[0043](1) Pass 120g of verapamil hydrochloride, 40g of pregelatinized starch, 30g of dextrin and 4g of talcum powder through a 100-mesh sieve, mix evenly, add 6g of starch slurry to make a suitable soft material, put the prepared soft material Put it into the extrusion material tank of the extrusion spheronizer, extrude the soft material, and spheronize to obtain a wet pill core, which is put into a fluidized bed for fluidization and drying to obtain a dry pill core for use;
[0044] Preparation of pellets:
[0045] (2) take by weighing 24g hypromellose sodium, add purified water under the situation of stirring, ultrasonically dissolve and make solution uniform, be made into the solution that weight percent concentration is 1%, as swelling layer coating solution; Take another step ( 1) The prepared drug-containing pellet core is placed in a fluidized bed, preheated for 15 min...
Embodiment 3
[0054]
[0055] Preparation:
[0056] The preparation of verapamil hydrochloride containing pill core:
[0057] (1) Pass 120g of verapamil hydrochloride, 52g of starch and 3g of micropowder silica gel through a 100-mesh sieve respectively, mix well, add 10g of polyvinylpyrrolidone to make a suitable soft material, put the prepared soft material into the extrusion spheronizer Extrude into the material tank, extrude the soft material, and roll it into a ball to obtain a wet pellet core, which is put into a fluidized bed for fluidization drying, and then the dry pellet core is obtained for use;
[0058] Preparation of pellets:
[0059] (2) Take 53g hypromellose E5 and add it into purified water while stirring, ultrasonically dissolve and make the solution uniform, and make a solution with a weight percent concentration of 3% as the swelling layer coating solution; another step ( 1) The prepared drug-containing pellet core is placed in a fluidized bed, preheated for 15 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com