Verapamil hydrochloride delayed-release capsule and preparation method thereof
A technology of verapamil hydrochloride and capsules, which is applied in the fields of cardiovascular system diseases, active ingredients of nitrile/isonitrile, active ingredients of heterocyclic compounds, etc., and can solve unfavorable industrialized large-scale production, difficult implementation of drug application process, and complicated overall process problems such as improved bioavailability, good reproducibility of absorption kinetics, and short preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
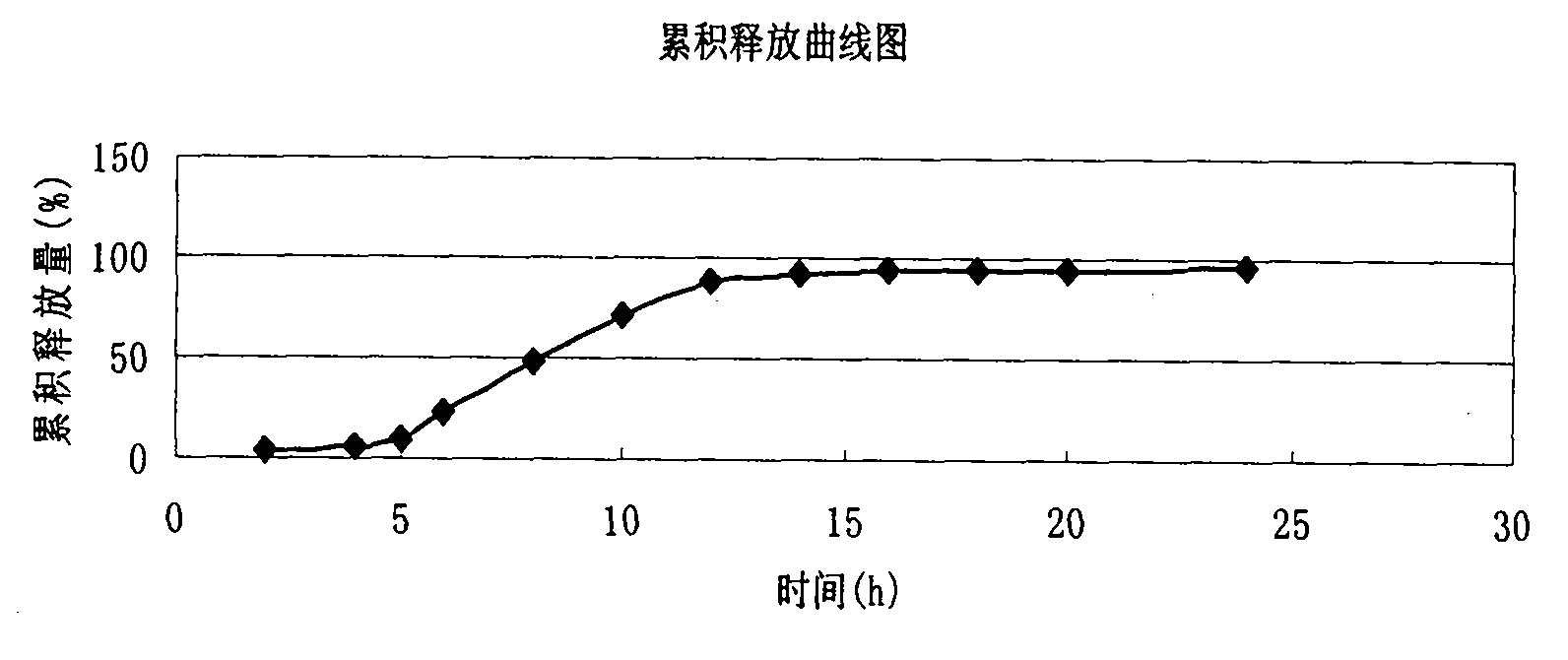
Embodiment 1
[0029] Microcrystalline cellulose (filler) 52g
[0030] Purified water (binder) 4g
[0031] Magnesium stearate (lubricant) 2g
[0032] Hypromellose E5 (swelling layer material) 42g
[0033] Acrylic resin (control release layer material) 84g
[0034] 1000 capsules in total
[0035] Preparation:
[0036] The preparation of verapamil hydrochloride containing pill core:
[0037] (1) Pass 120g of verapamil hydrochloride, 52g of microcrystalline cellulose and 2g of magnesium stearate through a 100-mesh sieve respectively, mix well, add 4g of purified water to make a suitable soft material, put the prepared soft material into the extruder Out of the extruded material tank of the spheronizer, the soft material is extruded and spheronized to obtain a wet pill core, which is put into a fluidized bed for fluidization drying to obtain a dry pill core for use;
[0038] Preparation of pellets:
[0039] (2) Take 42g hypromellose E5 and add it into purified water while stirring, ultr...
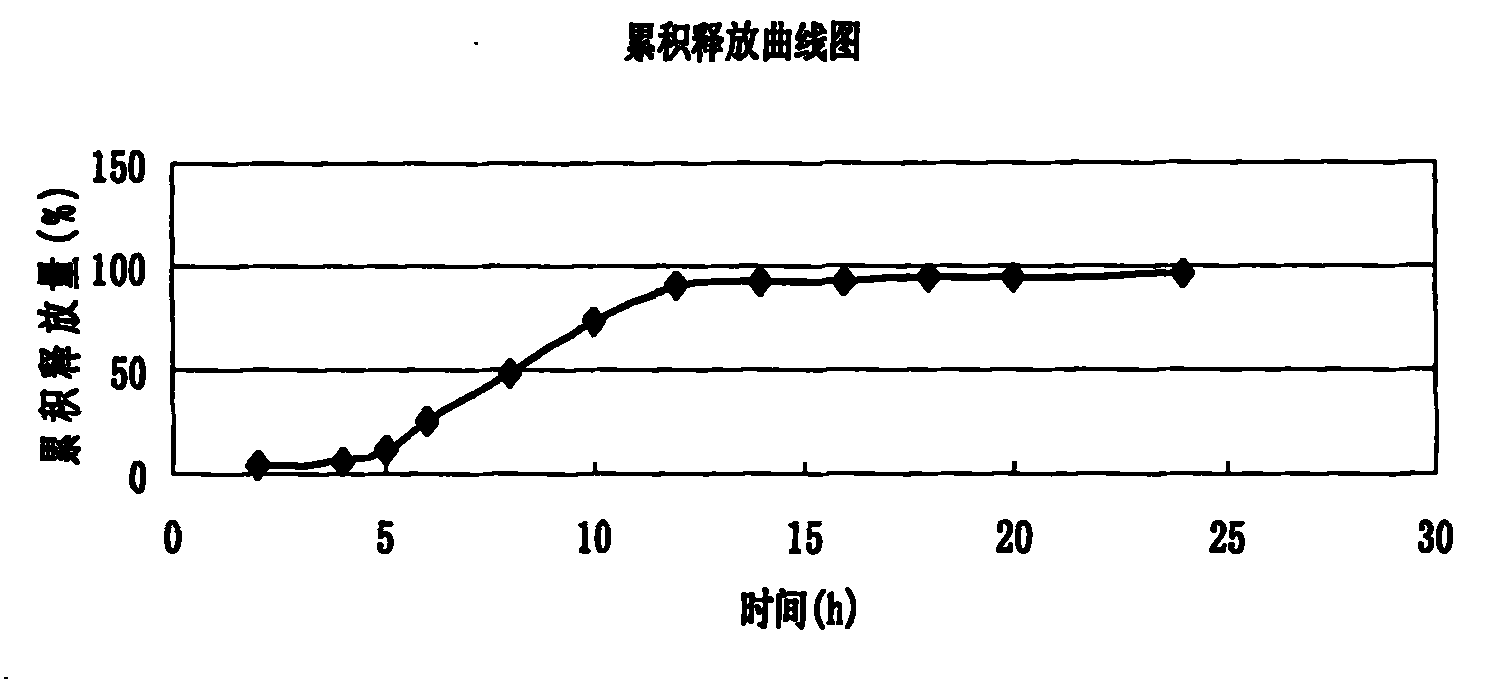
Embodiment 2
[0049] Pregelatinized starch (filler) 40g
[0050] Dextrin (filler) 30g
[0051] Starch slurry (adhesive) 6g
[0052] Talc powder (lubricant) 4g
[0053] Sodium hypromellose (swelling layer material) 24g
[0054] Ethylcellulose aqueous dispersion (solid content is 25%) (control release layer material) 284g
[0055] 1000 capsules in total
[0056] Preparation method: preparation of verapamil hydrochloride containing pill core:
[0057] (1) Pass 120g of verapamil hydrochloride, 40g of pregelatinized starch, 30g of dextrin and 4g of talcum powder through a 100-mesh sieve, mix evenly, add 6g of starch slurry to make a suitable soft material, put the prepared soft material Put it into the extrusion material tank of the extrusion spheronizer, extrude the soft material, and spheronize to obtain a wet pill core, which is put into a fluidized bed for fluidization and drying to obtain a dry pill core for use;
[0058] Preparation of pellets:
[0059] (2) take by weighing 24g hyp...
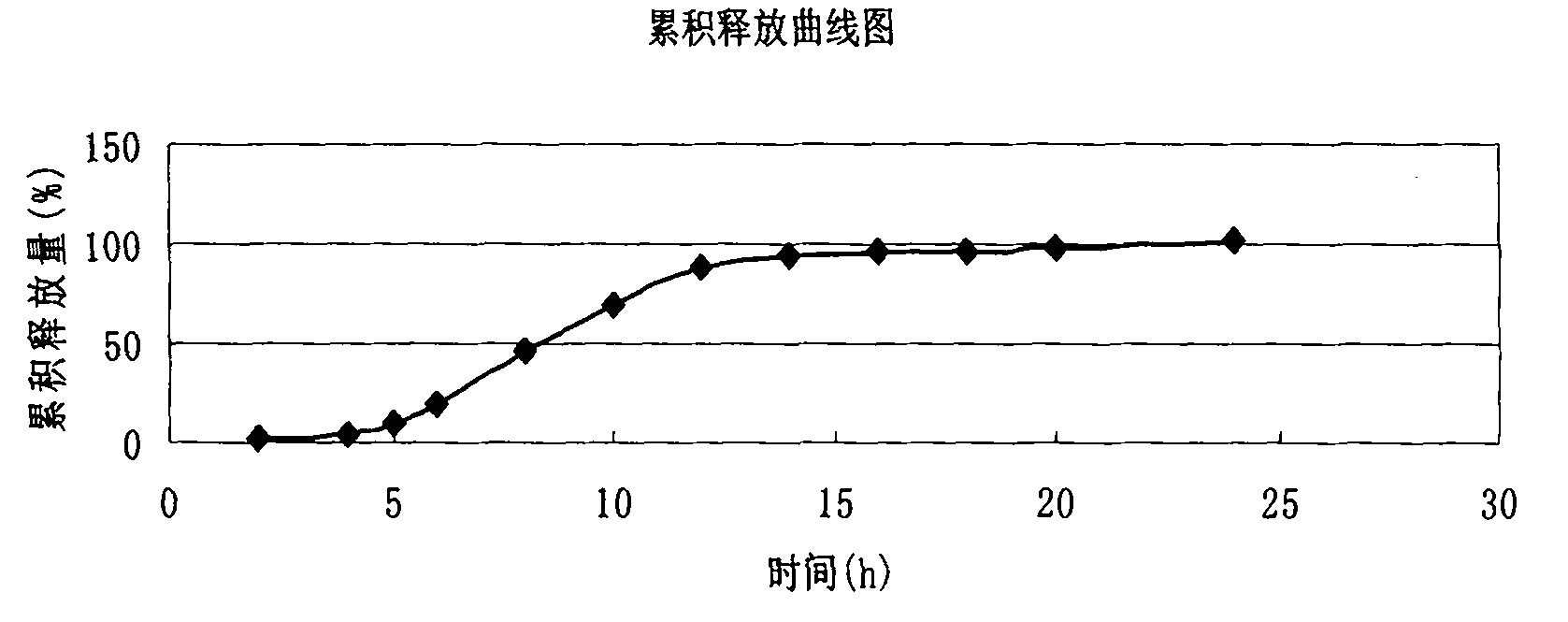
Embodiment 3
[0069] Starch (filler) 52g
[0070] Polyvinylpyrrolidone (adhesive) 10g
[0071] Micronized silica gel (lubricant) 3g
[0072] Hypromellose K40 (swelling layer material) 53g
[0073] Polyacrylic resin (control release layer material) 56g
[0074] 1000 capsules in total
[0075] Preparation:
[0076] The preparation of verapamil hydrochloride containing pill core:
[0077] (1) Pass 120g of verapamil hydrochloride, 52g of starch and 3g of micropowder silica gel through a 100-mesh sieve respectively, mix well, add 10g of polyvinylpyrrolidone to make a suitable soft material, put the prepared soft material into the extrusion spheronizer Extrude into the material tank, extrude the soft material, and roll it into a ball to obtain a wet pellet core, which is put into a fluidized bed for fluidization drying, and then the dry pellet core is obtained for use;
[0078] Preparation of pellets:
[0079] (2) Take 53g hypromellose E5 and add it into purified water while stirring, ul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com