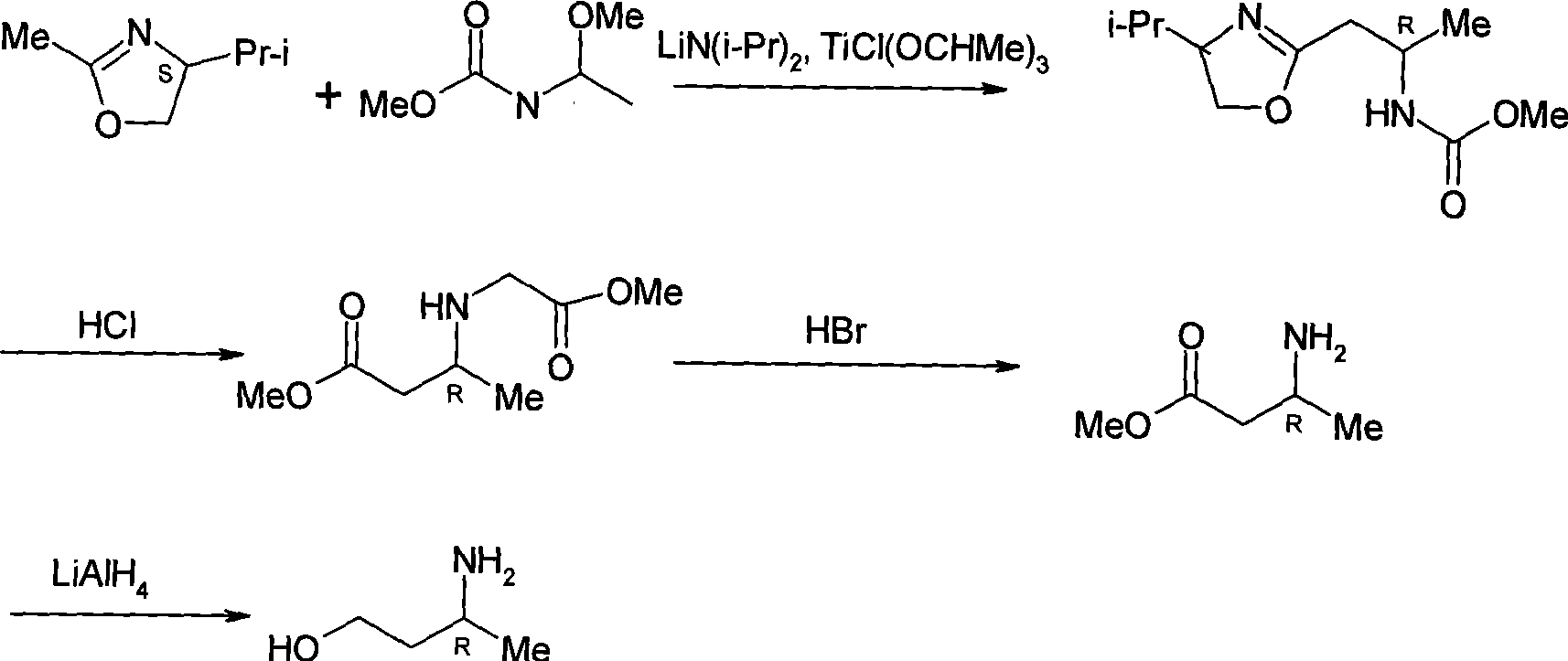
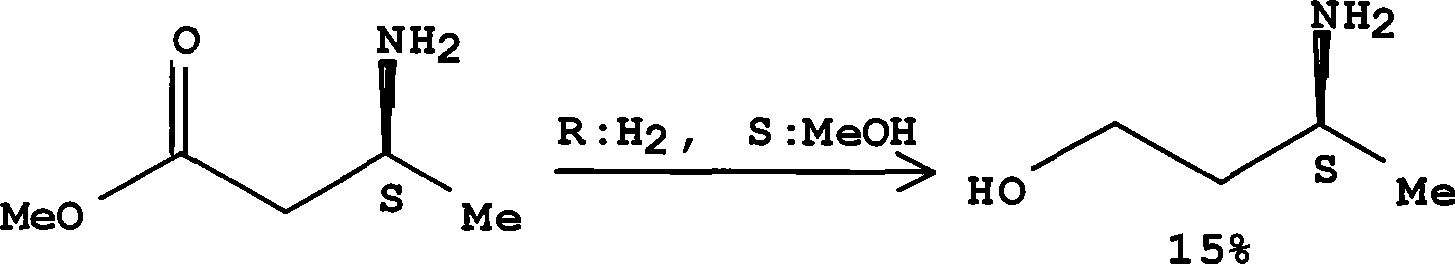Method for preparing optically pure 3-amino butyl alcohol
A technology of aminobutanol and aminobutyronitrile, which is applied in the field of preparation of chiral drugs, can solve problems such as unsuitable for industrial production, large loss in the resolution process, and inability to scale up production, etc., to reduce the difficulty of resolution, lower the cost, and increase the yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
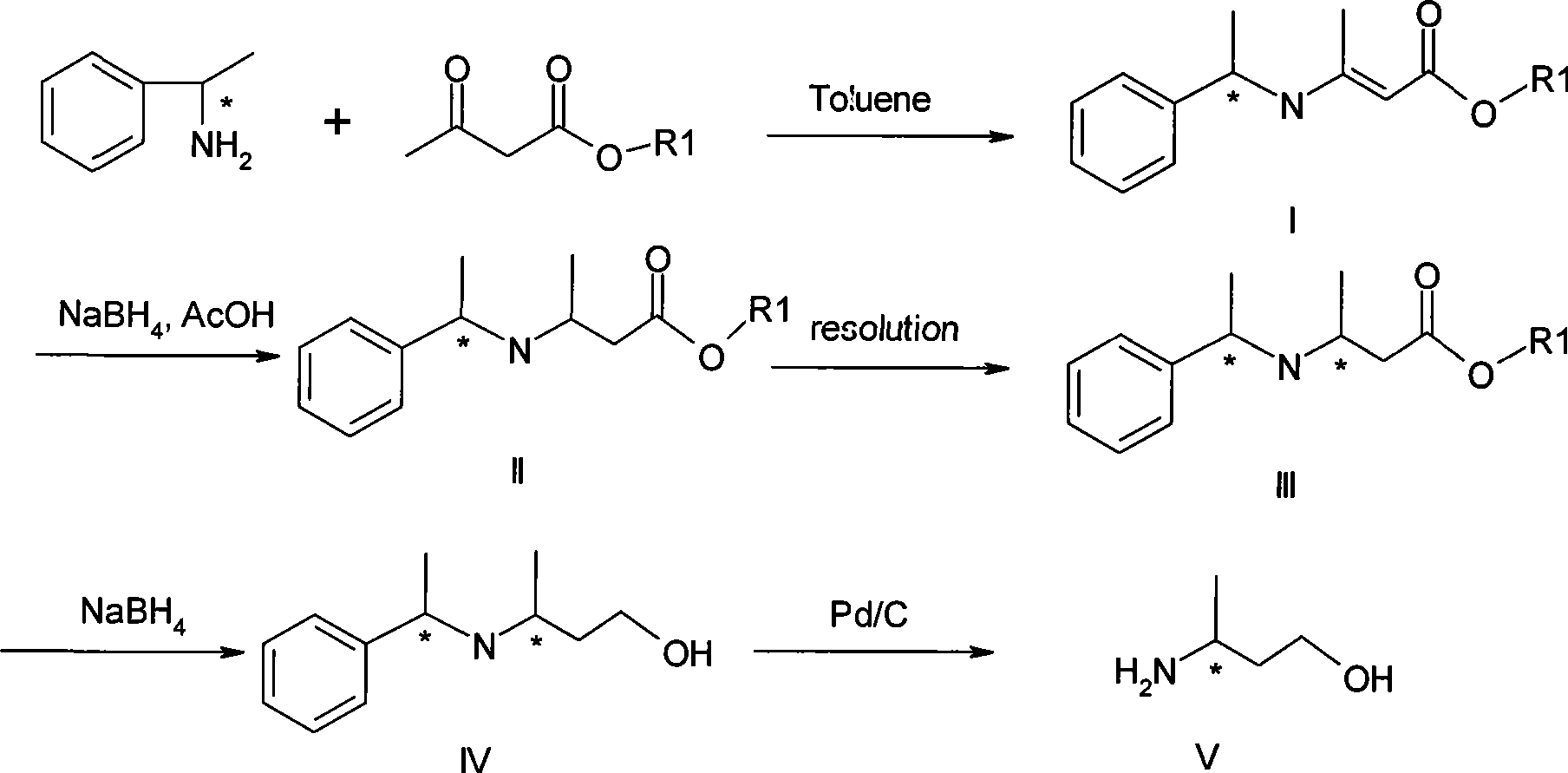
[0049] Embodiment 1.(R, R) and (R, S)-3-(1'-methylbenzylamine)-2-butenoic acid methyl ester preparation (I)
[0050] Add 62g methyl acetoacetate, 54g R-phenethylamine and 150ml toluene successively in a there-necked flask equipped with a thermometer, agitator and a water separator, heat, take toluene with water, and remove about 8g of water after the reaction ends, and the reaction After finishing, firstly evaporate toluene and ethyl acetoacetate on a rotary evaporator, and then evaporate a small amount of unreacted R-phenethylamine under 3-5mmHg to obtain (R, R) and (R, S)-3 -(1'-methylbenzylamine)-2-butenoic acid methyl ester 98g.
Embodiment 2
[0051] Example 2. Preparation of (1R, 2R) and (1R, 2S)-3-(1'-methylbenzylamine)-2-butenoic acid ethyl ester (I)
[0052] Add 58g of ethyl acetoacetate, 54g of R-phenethylamine and 150ml of toluene successively in a three-necked flask equipped with a thermometer, agitation and a water separator, heat, take toluene with water, and remove about 8g of water after the reaction ends, and the reaction After finishing, first evaporate toluene and ethyl acetoacetate on a rotary evaporator, then evaporate a small amount of unreacted R-phenethylamine under 3-5mmHg to obtain (1R, 2R) and (1R, 2S)-3 -(1'-methylbenzylamine)-2-butenoic acid ethyl ester 104g
Embodiment 3
[0053] Example 3. Preparation of (1S,2S) and (1S,2R)-3-(1'-methylbenzylamine)-2-butenoic acid methyl ester (I)
[0054] Add 62g methyl acetoacetate, 54g S-phenethylamine and 150ml toluene successively in a there-necked flask equipped with a thermometer, agitator and a water separator, heat, take toluene with water, and remove about 8g of water after the reaction ends, and the reaction ends Then, firstly, toluene and ethyl acetoacetate were evaporated on a rotary evaporator, and then a small amount of unreacted S-phenethylamine was evaporated at 3-5mmHg to obtain (1S, 2S) and (1S, 2R)-3- (1'-methylbenzylamine)-2-butenoic acid methyl ester 98g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com