Red luminous material for LED and producing process thereof
A red luminous, deep red technology, applied in the field of luminescent materials, can solve the problems of narrow emission peaks and limited applications, and achieve the effects of low equipment requirements, low raw material costs, and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
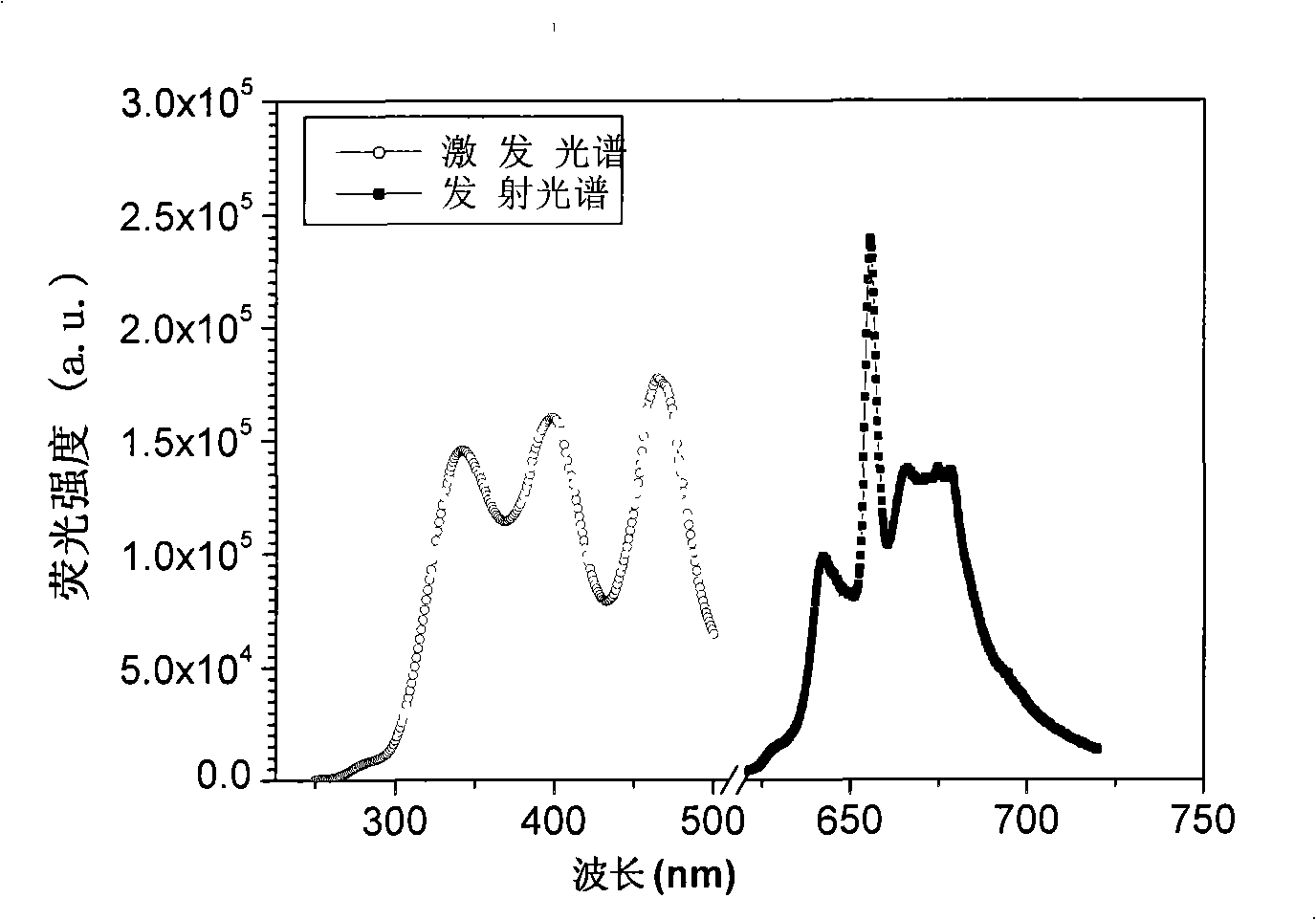
[0018] According to target product Ca 1-x-y Sr x Mg y Al 12-z o 19 :Mn 4+ (wherein x=0, y=0, z=0.01%) each ion stoichiometric ratio, accurately measure the above Ca(Ac) 2 , Al(NO 3 ) 3 , Mn(Ac) 2 After mixing the solution evenly, add urea whose molar mass is 3 times the total molar amount of metal ions, stir to dissolve it, and form a transparent viscous solution, place it on an electric furnace, heat and concentrate until it burns, and obtain a yellow precursor powder. After pre-burning in the air at 500°C for 5 hours, grind it, then sinter in the air at 1300°C for 5 hours, and cool it down to room temperature naturally to get the product. Fluorescent red.
example 2
[0020] According to target product Ca 1-x-y Sr x Mg y Al 12-z o 19 :Mn 4+ (wherein x=0.5, y=0, z=0.1%) each ion stoichiometric ratio, accurately measure the above Ca(Ac) 2 , Sr(NO 3 ) 3 , Al(NO 3 ) 3 , Mn(Ac) 2 After mixing the solution evenly, add citric acid whose molar amount is 3 times the total molar amount of metal ions, stir to dissolve it, form a transparent viscous solution, place it on an electric furnace, heat and concentrate until it burns, and obtain a brown precursor powder. The product is pre-fired in the air at 600°C for 8 hours, then ground, then sintered in the air at 1100°C for 24 hours, and cooled to room temperature naturally to obtain the product. The product emits light under the irradiation of ultraviolet lamp, ultraviolet LED or blue light LED Crimson fluorescent.
example 3
[0022] According to target product Ca 1-x-y Sr x Mg y Al 12-z o 19 :Mn 4+ (wherein x=0, y=0.5, z=0.05%) each ion stoichiometric ratio, accurately measure the above Ca(Ac) 2 , Mg(NO 3 ) 3 , Al(NO3 ) 3 , Mn(Ac) 2 Solution, after mixing evenly, add citric acid and urea with a molar mass of 1.5 times the total molar amount of metal ions, stir to dissolve, form a transparent viscous solution, place it on an electric furnace, heat and concentrate until it burns, and obtain a brown precursor powder , pre-burn the precursor in the air at 500°C for 5 hours, grind it, and then sinter it in the air at 1100°C for 18 hours, and cool it to room temperature naturally to get the product. The product is irradiated by ultraviolet lamp, ultraviolet LED or blue LED Underneath, it fluoresces dark red.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com