Synthesis of oligomerized fragrant amide-containing paramagnetic metallo-chelates contrast medium
A paramagnetic metal and synthesis method technology, applied in the fields of chemistry and medicine, can solve the problems of poor imaging effect, short residence time and high osmotic pressure of hepatic and biliary tract systems, achieve good stability and relaxation rate, and improve early diagnosis Level, enhance the effect of relaxation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
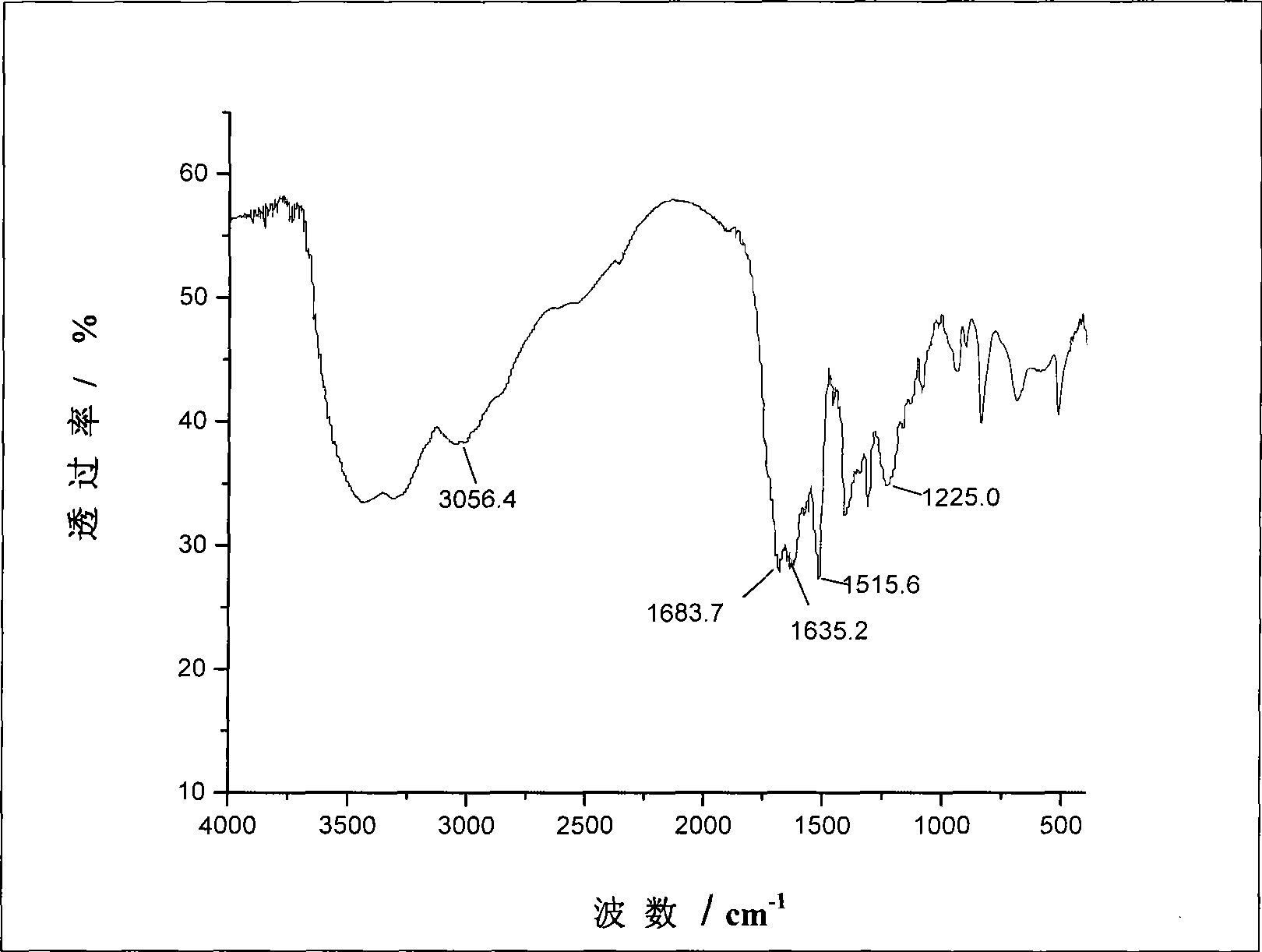
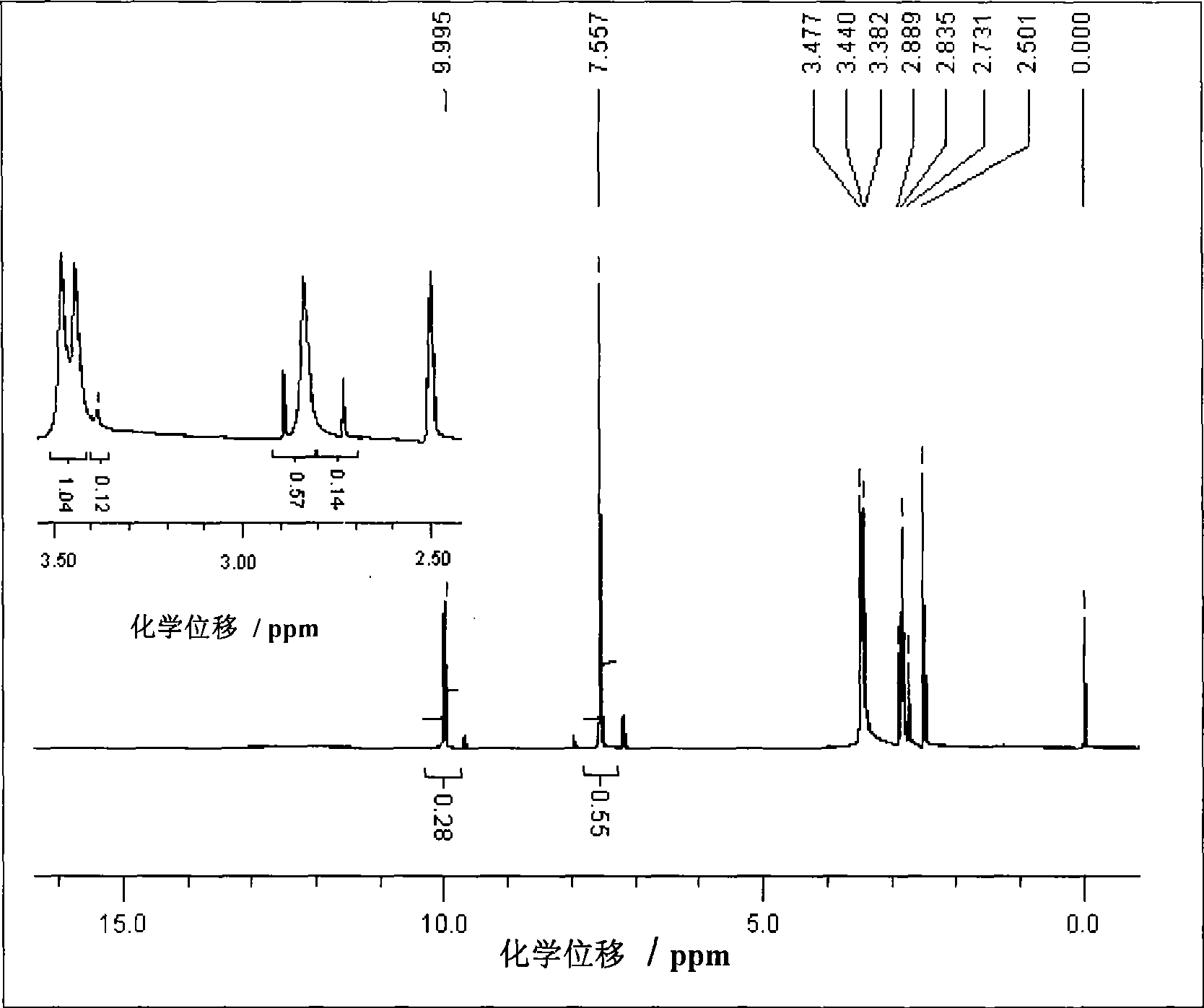
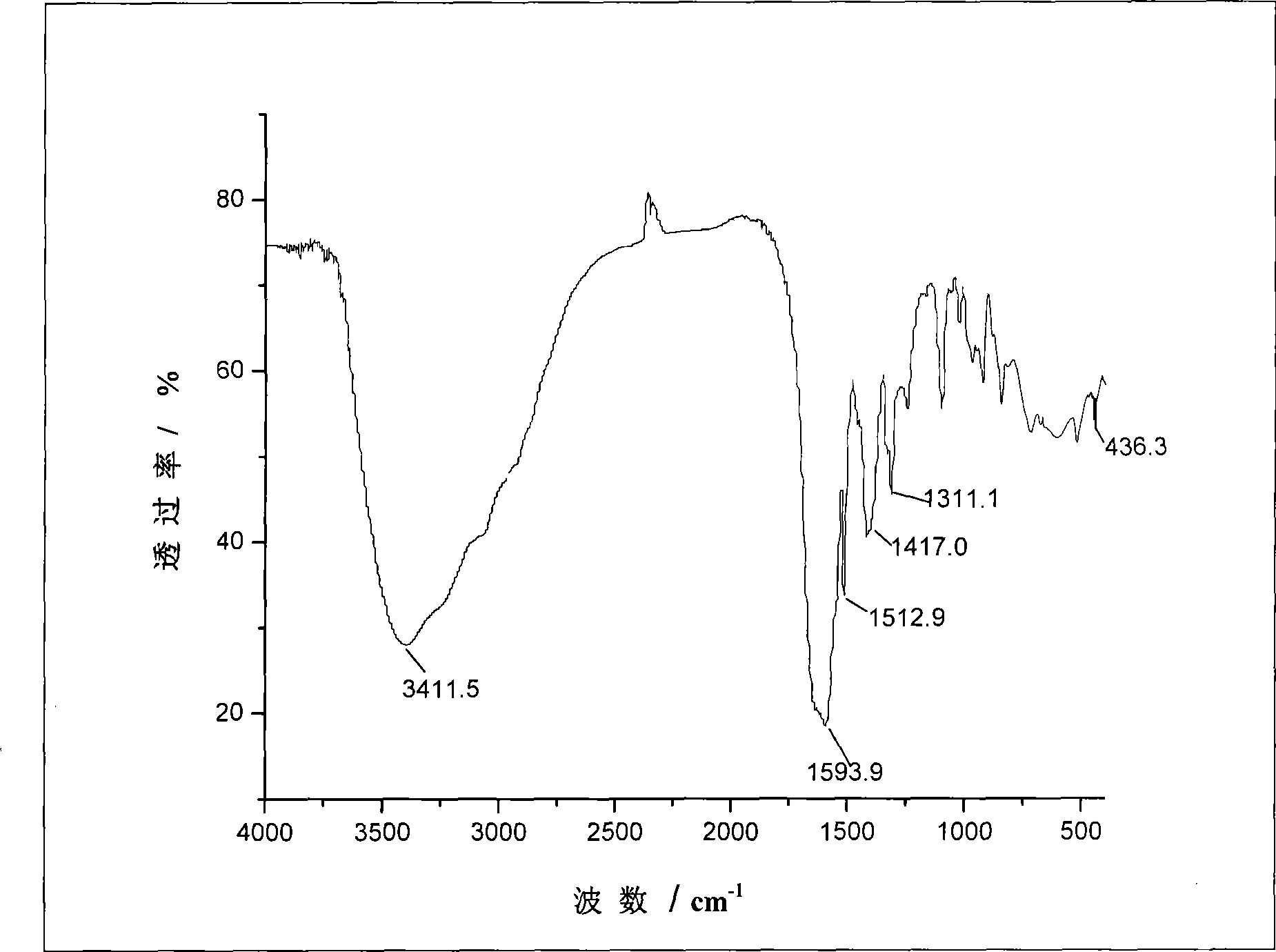
Image
Examples
Embodiment 1
[0042] Oligoethylenediamine-N,N'-bis(acetanilide)-gadolinium(III) diacetate chelate
[0043] The preparation of step 1.ethylenediaminetetraacetic dianhydride (EDTAA):
[0044] Weigh 5.80g (0.02mol) of ethylenediaminetetraacetic acid (EDTA) into a 50mL round bottom flask, add 8mL (0.08mol) of acetic anhydride and 10mL (0.12mol) of pyridine, and install a straight condenser with a drying tube above the flask Stir and reflux at 65°C for 24h, cool to room temperature, filter with suction, wash with acetic anhydride, cold DMF and ether, recrystallize with DMF-ether, and dry in vacuo to obtain a white powder called ethylenediaminetetraacetic dianhydride (EDTAA), yield 74%. m.p. is 189-191°C; elemental analysis measured value (%, calculated value): C 46.51 (46.87), H 4.98 (4.69), N 10.27 (10.94).
[0045] Step 2. Preparation of oligoethylenediamine-N, N'-bis(acetanilide)-diacetic acid:
[0046] 10.81g (0.1mol) p-phenylenediamine, 25.63g (0.1mol) ethylenediaminetetraacetic dianhydr...
Embodiment 2
[0050] Oligomeric diethylenetriamine-N,N'-bis(acetanilide)-triacetate gadolinium(III) chelate
[0051] The preparation of step 1. diethylenetriaminepentaacetic dianhydride (DTPAA):
[0052] Weigh 7.80g (0.02mol) of diethylenetriaminepentaacetic acid (DTPA) into a 50mL round bottom flask, add 8mL (0.08mol) of acetic anhydride and 10mL (0.12mol) of pyridine, and install a straight drying tube on the top of the flask Condenser, stirred and refluxed at 65°C for 24 hours, cooled to room temperature, filtered with suction, washed with acetic anhydride, cold DMF and ether, recrystallized with DMF-ether, and dried in vacuo to obtain white powder as diethylenetriaminepentaacetic acid Dianhydride (DTPAA), yield 78%. m.p. is 182-184°C; elemental analysis measured value (%, calculated value): C 47.51 (47.06), H 5.47 (5.32), N 12.03 (11.76).
[0053] Step 2. Oligomeric diethylenetriamine-N,N'-bis(acetanilide)-triacetic acid:
[0054] Put 10.81g (0.1mol) p-phenylenediamine, 35.73g (0.1mo...
Embodiment 3
[0058] Oligoethylenediamine-N,N'-bis(acetylbenzidine)-gadolinium(III) diacetate chelate
[0059] The preparation of step 1.ethylenediaminetetraacetic dianhydride (EDTAA):
[0060] With embodiment 1.
[0061] Step 2. The preparation of oligoethylenediamine-N, N'-bis(acetylbenzidine)-diacetic acid:
[0062] Put 18.40g (0.1mol) p-diaminobiphenyl into the three-necked flask, 25.63g (0.1mol) ethylenediaminetetraacetic dianhydride (EDTAA), then add 25mL N-methylpyrrolidone (NMP), 15mL pyridine, and nitrogen , after stirring and reacting at 30° C. for 5 h, the mixed solution was dropped into rapidly stirred distilled water to precipitate a precipitate, which was filtered to obtain a solid. Then the solid was dissolved with an appropriate amount of N-methylpyrrolidone (NMP), filtered, and the filtrate was dropped into rapidly stirred distilled water. This was repeated three times and dried in vacuo to obtain a white powder of oligoethylenediamine-N, N'-bis( Acetylbenzidine)-diaceti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com