Astragalus polysaccharide adjuvant and uses in type I riemerella anatipestifer inactivated vaccine
The technology of astragalus polysaccharide and Reesei bacteria is applied in the field of vaccine immune adjuvant and vaccine, which can solve the problem that duck infectious serositis has no immune effect, and achieve the prevention of duck infectious serositis, good safety and immunogen Sex, the effect of improving the immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
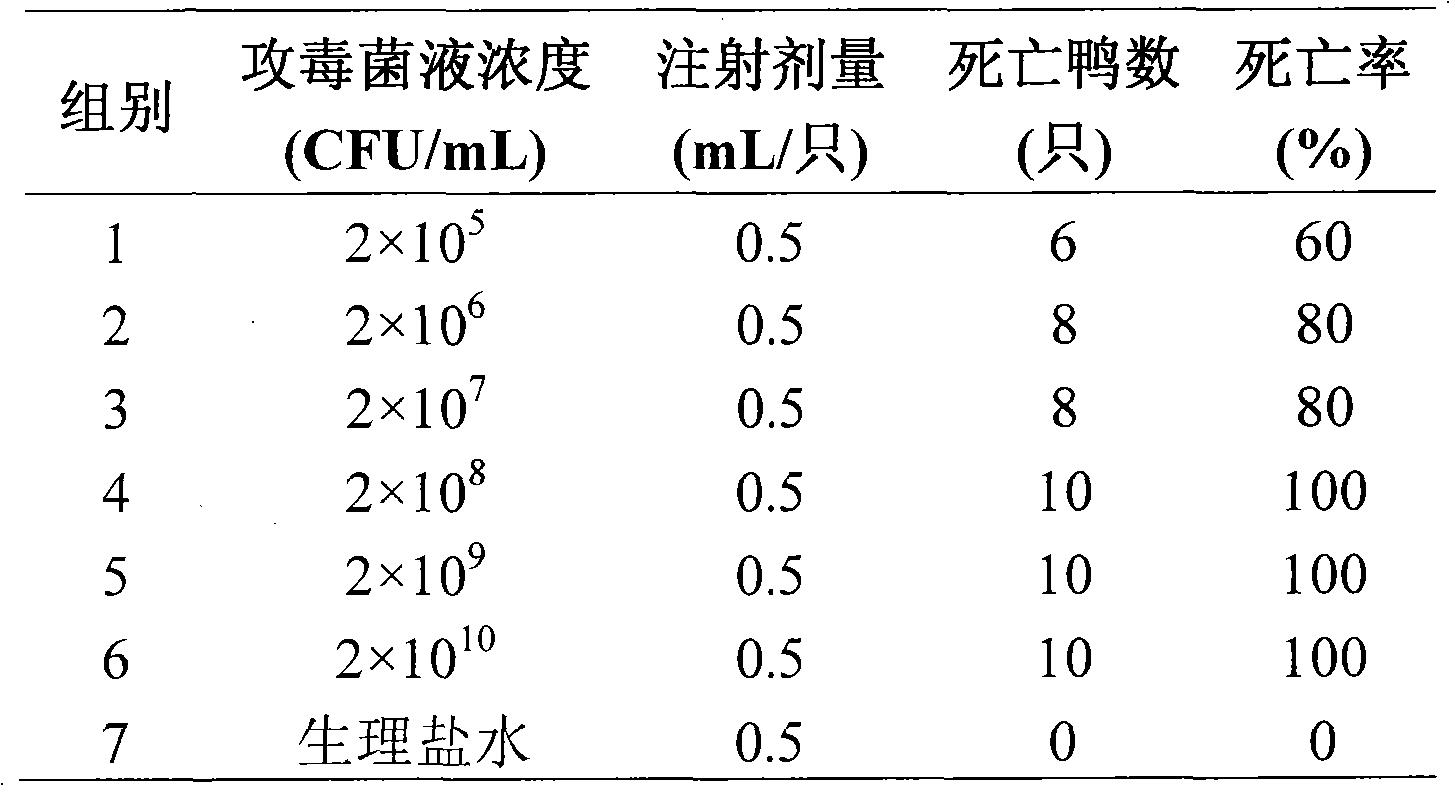
Image
Examples
Embodiment 1
[0021] (1) Inoculate the R. anatipestifer RA-1 strain on a TSA plate and inoculate it in TSB, culture it with shaking at 37°C for 18-24 hours, take the culture solution and inoculate it on TSA and blood agar plates, and use it as seeds after passing the purity inspection. solution, stored at 4°C;
[0022] (2), seed liquid is inoculated in TSB by 5% of the culture liquid volume, 37 DEG C ventilated and shakes and cultivates for 24 hours, adopts the plate counting method to carry out bacterial count after the pure inspection is qualified, simultaneously adds formaldehyde by 0.4% of the bacterial liquid volume The solution was inactivated at 37°C for 24 hours, and after inactivation, the concentration of the bacterial solution was adjusted to 1×10 10 CFU / mL, inoculated on blood agar and TSA plates, 5% CO 2 , Cultivate at 37°C for 48 hours, and those with aseptic growth are used to prepare vaccines;
[0023] (3), astragalus polysaccharide is made into the solution that concentra...
Embodiment 2
[0026] Step (1), (2) are identical with embodiment 1;
[0027] (3), astragalus polysaccharide is made into the solution that concentration is 20mg / ml;
[0028] (4), mix the inactivated bacterium liquid of R. anatipestifer RA-1 and the astragalus polysaccharide solution in a ratio of 1:1;
[0029] (5) Add 0.01% thimerosal of the total volume of the mixed solution to the mixed solution.
[0030] Materials and methods used in the present invention and the immune effect experiment are further described below:
[0031] 1 Materials and methods
[0032] 1.1 Materials
[0033] 1.1.1 Strains
[0034] The type I RA strain was isolated from a meat duck farm in the suburbs of Wuhan, and was freeze-dried after identification.
[0035] 1.1.2 Medium
[0036] Tryptic Soy Agar (Tryptic Soy Agar, TSA) and Tryptic Soy Broth (Tryptic Soy Broth, TSB) are products of Difco and Bacto, respectively, and 10% calf serum was added when used.
[0037] 1.1.3 Adjuvant raw materials
[0038] Astraga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com