Orodispersible domperidone tablets
A technology of domperidone and dispersible tablets, which is applied in the direction of organic active ingredients, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as tablet breakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 (domperidone tablet)
[0073] step 1:
[0074] In a planetary or paddle mixer, put the following compounds:
[0075] — Mannitol 60
[0076] -Domperidone
[0077] — Maltodextrin
[0078] - croscarmellose sodium (50% of the total amount)
[0079] mix
[0080] Step 2:
[0081] In a container of sufficient capacity, add:
[0082] -pure water
[0083] Slowly pour purified water over the moving mixture.
[0084] Mix until a suitable granulation is obtained.
[0085] Step 3:
[0086] The wet mass obtained in step 2 was pulverized with a vibrating granulator equipped with an 8 mm screen.
[0087] The crushed particles were collected in a tared fluid bed tank.
[0088] Step 4:
[0089] Dry the wet granules in a fluidized bed until the residual moisture is less than or equal to 2%.
[0090] Step 5:
[0091] The following materials were size classified using a vibrating granulator fitted with a 1 mm screen:
[0092] — dry granules
[0093] — Peppermi...
Embodiment 2
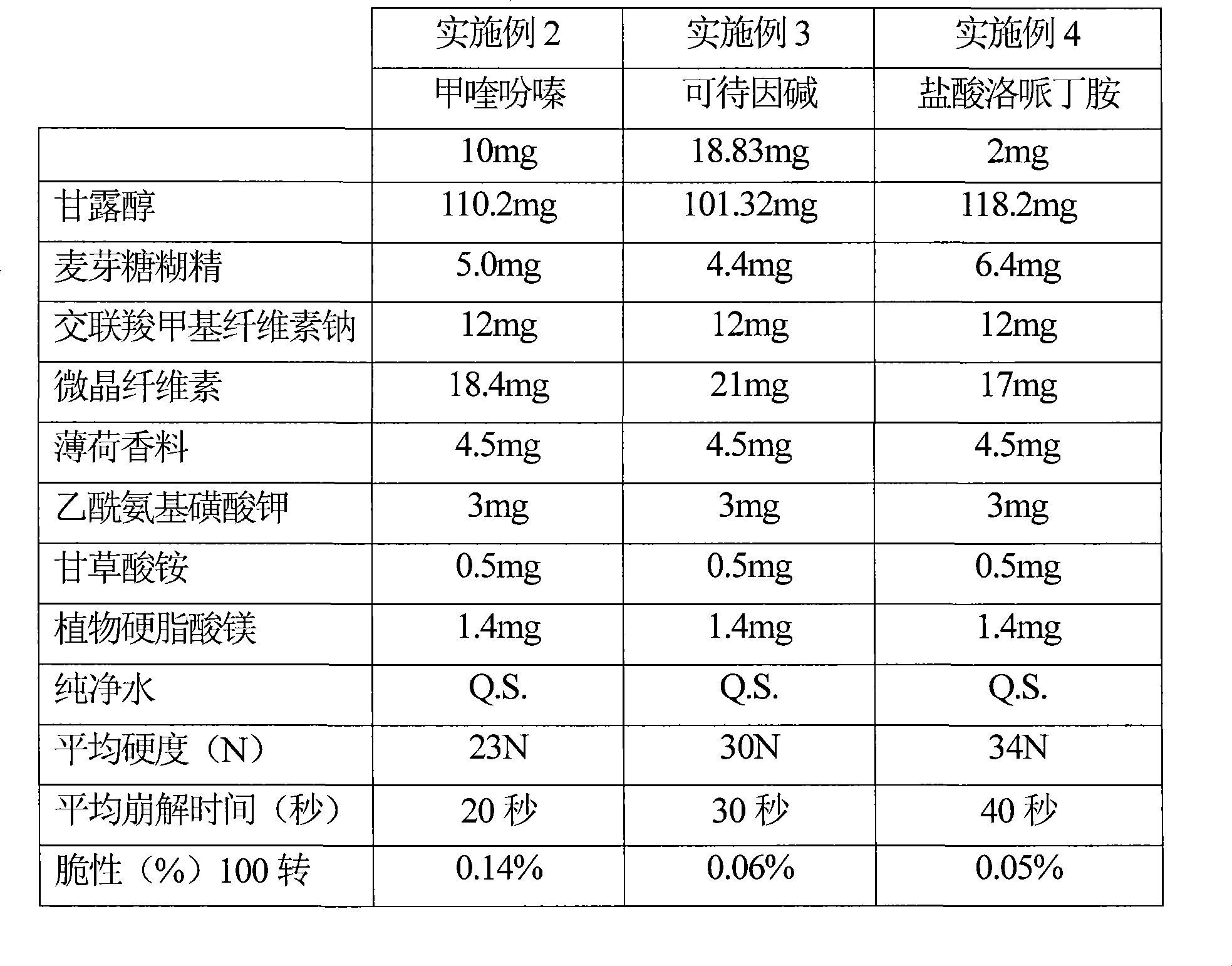
[0116] Example 2: Tablets Containing Mequinazine
Embodiment 3
[0117] Example 3: Tablets containing codeine base
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com