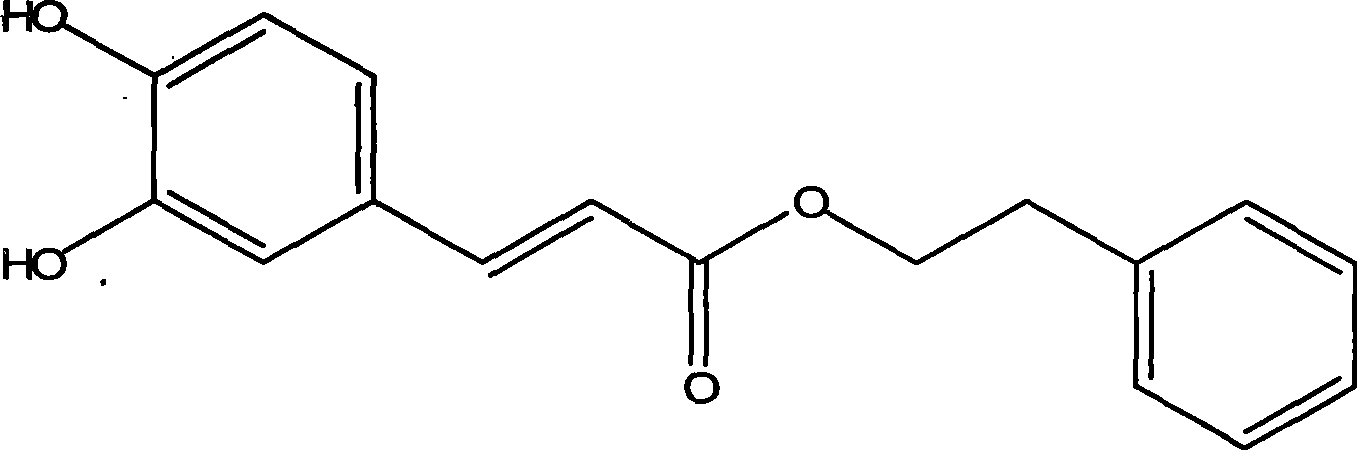
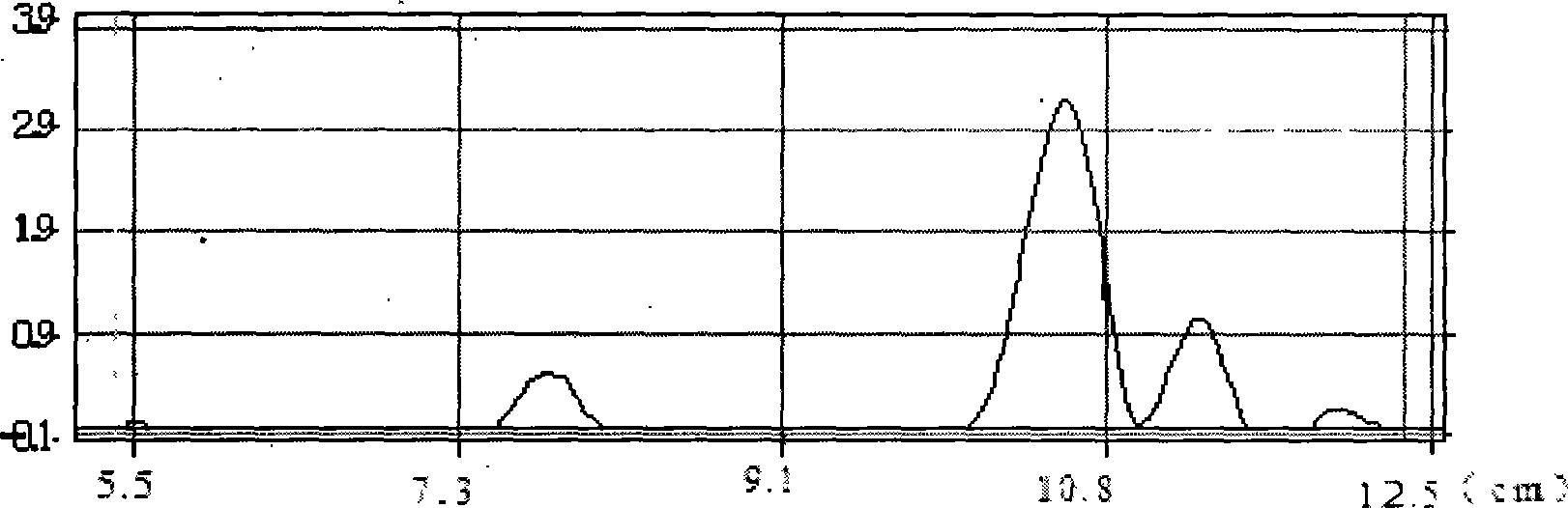
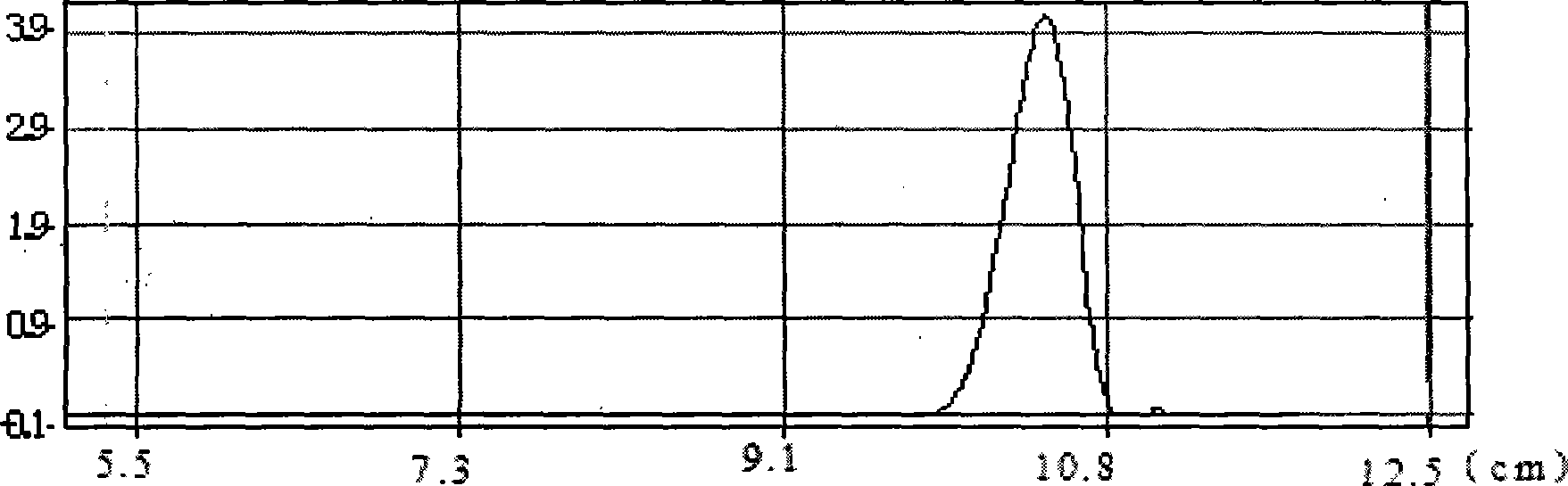
Method for enzymatic synthesis of caffeic acid phenethyl ester by solvent-free system
A caffeic acid phenethyl ester, enzymatic synthesis technology, applied in the direction of fermentation, etc., can solve the problems of chemical synthesis method, such as many by-products, destroying the structure of natural compounds, long extraction time, etc., to facilitate industrial production, avoid environmental pollution, reaction The effect of conditional safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Get 50 grams of phenylethyl alcohol and 10 grams of Molecular sieves were placed in a 100mL Erlenmeyer flask with a stopper, placed in an air shaker at 50°C, fully shaken at 180rpm for 48 hours, and filtered to remove the molecular sieves to obtain anhydrous phenylethanol;
[0026] (2) Enzyme-catalyzed reaction Add 2.0mmoL caffeic acid, 184mmoL phenylethyl alcohol, 120mg Novozym435 lipase into a 50mL Erlenmeyer flask with a stopper, and react in an oil bath at 70°C for 48h with oscillation at 50r / min;
[0027] (3) After the reaction was stopped, the unreacted phenethyl alcohol was removed by rotary evaporation and purified. Purification conditions: Packing: Take 25-30g of silica gel G, and use n-hexane as solvent to wet-pack the column, and control the flow rate to 1 drop / s. Chromatography conditions: adopt the method of stepwise elution, control the flow rate of the eluent ratio for 5 minutes and connect the effluent, gradually increase from benzene: ether = 10:3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com