Separation and purification method of haloperoxidase
A technology of halogen peroxide and peroxidase, which is applied in the direction of biochemical equipment and methods, enzymes, enzymes, etc., can solve the problem of less research on physiological functional proteins of red algae, increase the difficulty of enzyme separation and purification, and affect the detection of enzyme activity, etc. problems, to achieve the effect of low cost, easy operation and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
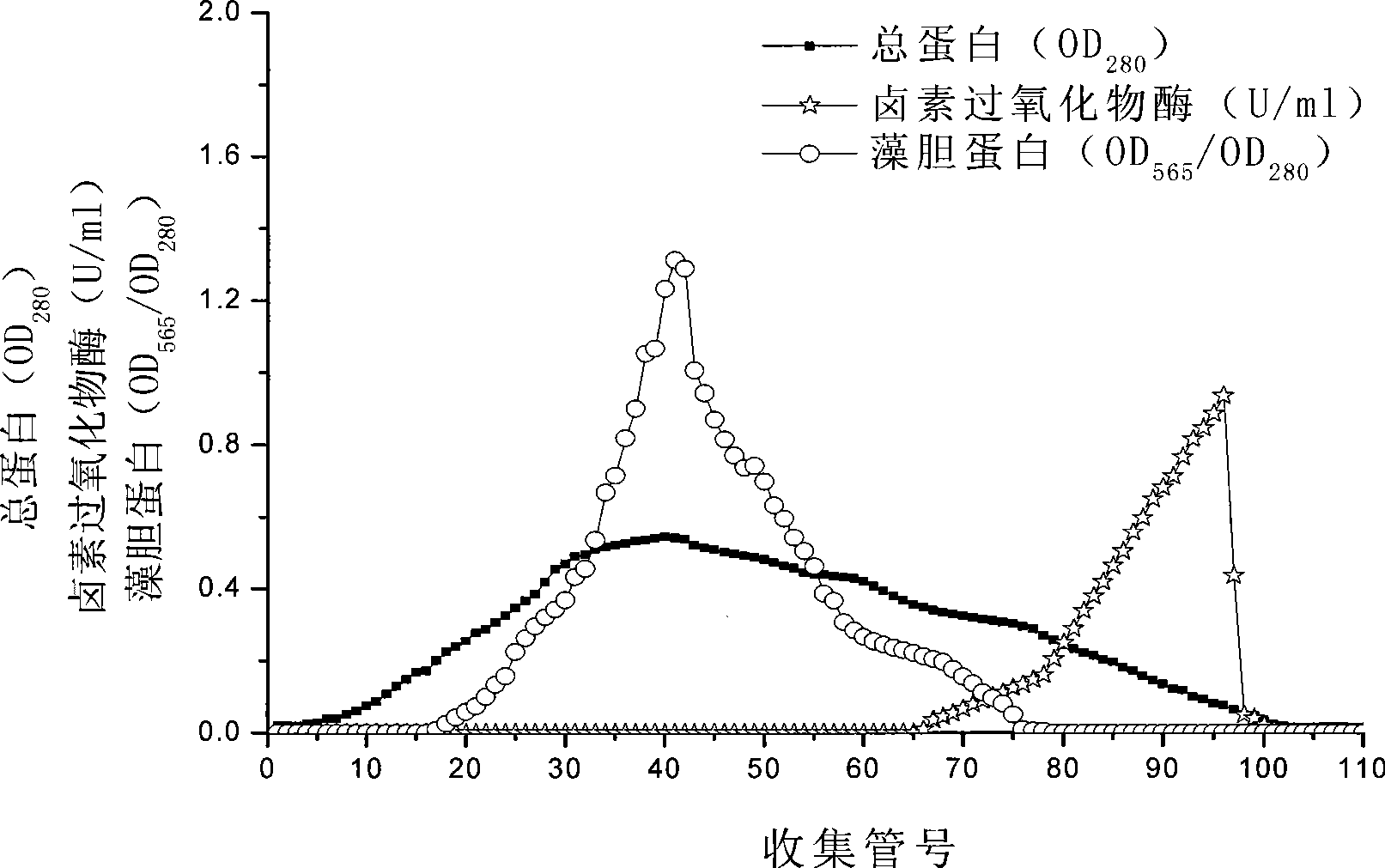
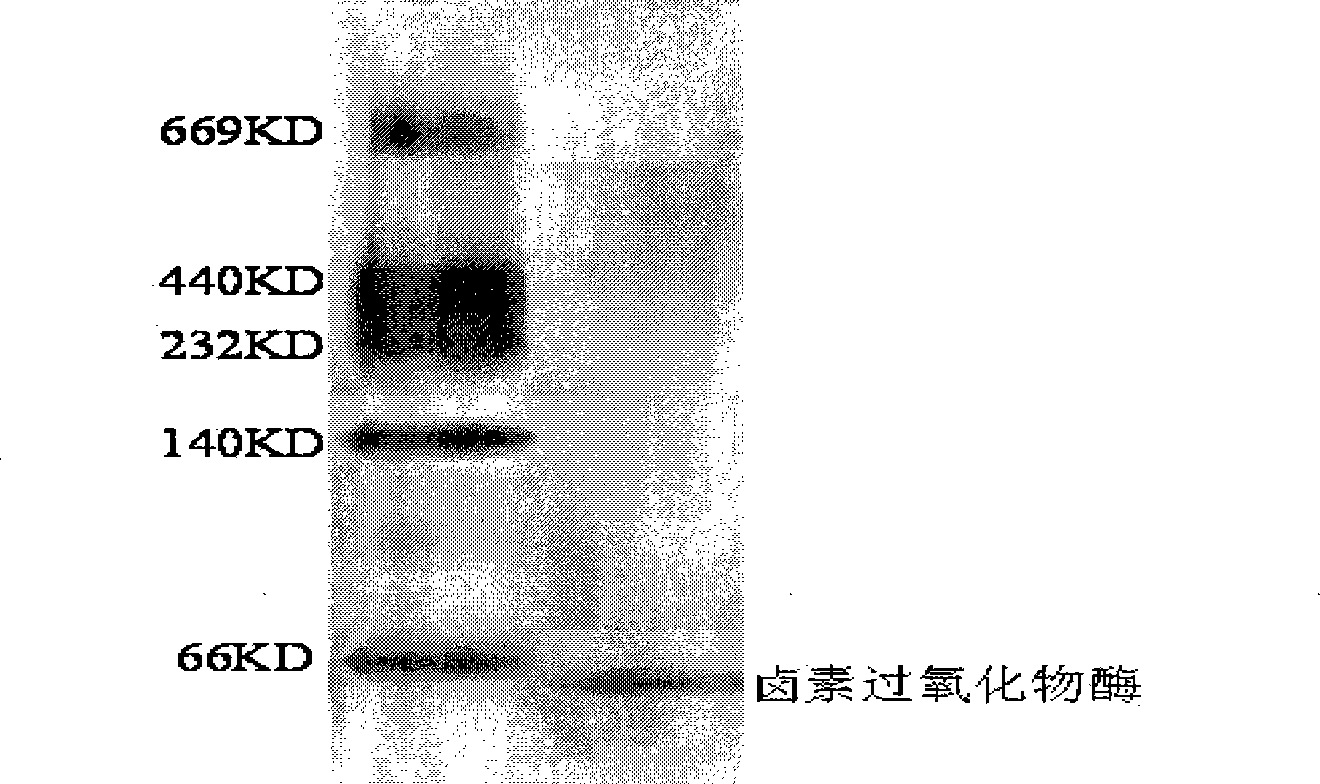
[0026] Take 200g wet weight of Asparagus algae, grind it into powder with liquid nitrogen; add 200ml Tris-HCl buffer solution (0.02mol / L, pH6.5), soak at 0-5°C for 30h, and filter with gauze. Centrifuge at 4000g for 15min at 4°C, and take the supernatant. Crude extract plus solid (NH 4 ) 2 SO 4 To 15% of the final mass concentration, centrifuge at 7000g for 20min at 4°C. The precipitate was discarded, and the supernatant was used again (N H 4) 2 SO 4 Precipitate (65% of mass concentration), centrifuge at 10000g for 15min at 4°C. The precipitate was collected and dialyzed with 0.02mol / L, pH6.5 Tris-Cl buffer solution for 15 hours to obtain a crude enzyme solution of haloperoxidase; the molecular weight cut-off of the dialysis membrane was 14000. The dialyzed enzyme liquid sample was separated on a DEAE-sepharose column, and 500ml of Tris-HCl buffer containing NaCl concentration varied from 0.05 to 1.0M was eluted with a linear gradient of 0.02mol / L, pH 6.5. Collect at i...
Embodiment 2
[0032] Take 400 grams of Asparagus algae with a wet weight, grind it into powder with liquid nitrogen; add 300ml Tris-HCl buffer solution (0.08mol / L, pH8.5), soak at 6-8°C for 65h, and filter with gauze. Centrifuge at 7000g for 40min at 8°C, and take the supernatant. Crude extract plus solid (NH 4 ) 2 SO 4 To 25% of the final mass concentration, centrifuge at 14000g for 40min at 8°C. The pellet was discarded, and the supernatant was washed again with NH 4 ) 2 SO 4 Precipitate (85% of mass concentration), centrifuge at 15000g for 30min at 8°C. The precipitate was collected and dialyzed with 0.08mol / L, pH8.5 Tris-Cl buffer for 48 hours to obtain a crude enzyme solution of haloperoxidase; the molecular weight cut-off of the dialysis membrane was 14,000. The dialyzed enzyme solution sample was separated on a DEAE-cellulose column, and 500ml of Tris-HCl buffer solution containing NaCl concentration varied from 0.1 to 2.0M with a linear gradient of 0.08mol / L and pH8.5 was use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com