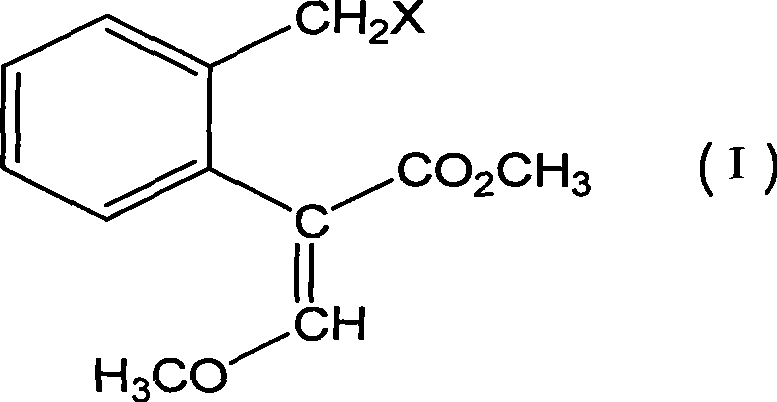
Bactericidal agent intermediate (E)-2-(2'-bromomethyl)phenyl-3-methoxylacrylate preparation method
A technology of methyl methoxyacrylate and o-methylphenylacetic acid, which is applied in the field of preparation of formula intermediates, can solve the problems of low reaction yield, difficult market source of isochromanone, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
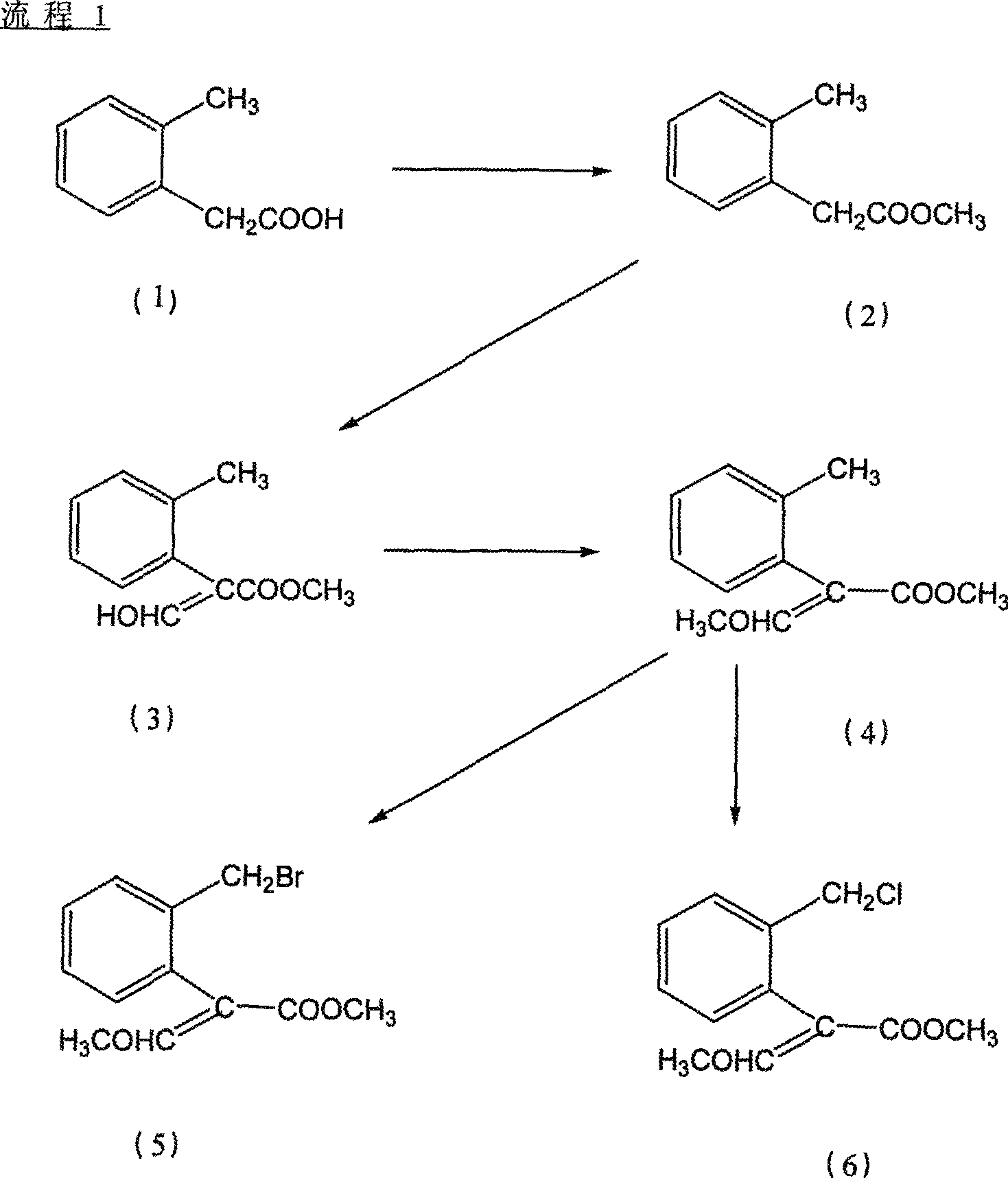
[0027] Embodiment 1, the preparation method of agricultural fungicide chemical intermediate (E)-2-(2'-bromomethyl)phenyl-3-methoxymethyl acrylate, with reference to Fig. 1:
[0028] step 1)
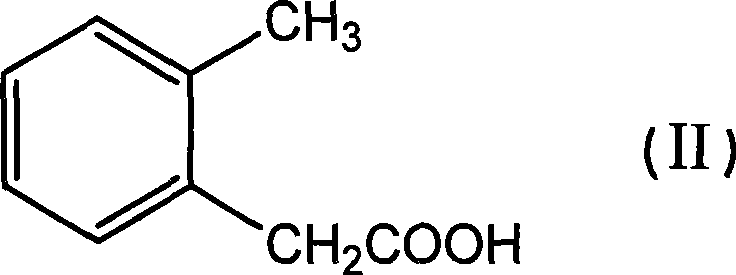
[0029] Preparation of methyl o-tolyl acetate
[0030] Add 151.7g (1.0mol, 99%) o-methylphenylacetic acid into a 1000ml reaction bottle, add 500ml methanol to dissolve under stirring, add 25ml concentrated sulfuric acid, heat and reflux for 6-12 hours, evaporate the solvent under reduced pressure after cooling, and distill The product was extracted with toluene, washed with water three times, dried with anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure, and the product was evaporated under reduced pressure to obtain 162.1g, a colorless liquid with a content of 99.3% and a yield of 98%.
[0031] 1 H-NMR (CDCl 3 , TMS) δ: 7.21-7.01 (m, 4H), 3.61 (s, 3H), 3.60 (s, 2H), 2.35 (s, 3H); MS (m / e): 164 (M + , 42), 133(100), 31(82).
[0032] Step 2)
[0033] Prepar...
Embodiment 2
[0050] Embodiment 2 is basically the same as Embodiment 1, but step 4 wherein is changed to:
[0051] Preparation of (E)-2-(2'-chloromethyl)phenyl-3-methoxymethyl acrylate
[0052] 21g (0.1mol, 98%) 2-(2'-methyl) phenyl-3-methoxymethyl acrylate, 0.42g (2.5mmol, 99%) AIBN and 60ml benzene drop into 100ml reaction bottle, heat up 80 ℃, slowly feed chlorine gas, and follow up analysis by gas chromatography. After 2 hours, the chlorine feed is completed. The solvent is distilled off on a rotary evaporator, purified by a chromatographic column, and eluted with n-hexane / ethyl acetate = 4 / 1 to obtain 18.25 g(E)-2-(2'-chloromethyl)phenyl-3-methoxymethyl acrylate, a light yellow colloid.
[0053] 1 H-NMR (CDCl 3 , TMS) δ: 7.61 (s, 1H), 7.5-7.0 (m, 4H), 4.49 (s, 2H), 3.80 (s, 3H), 3.69 (s, 3H);
[0054] MS(m / e): 240(M + , 10), 210(12), 196(21), 149(38), 129(100).
Embodiment 3
[0055] Embodiment 3 is basically the same as Embodiment 1, but has the following changes:
[0056] The catalyst of step 1) is changed into: p-toluenesulfonic acid;
[0057] Add 151.7g (1.0mol, 99%) of o-toluene acetic acid to a 1000ml reaction flask, add 500ml of methanol to dissolve under stirring, add 26.4g (0.15mol, 98%) of p-toluenesulfonic acid, heat and reflux for 6-12 hours, After cooling, the solvent was evaporated under reduced pressure, the residue was extracted with toluene, washed with water three times, dried with anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure, and the product was evaporated under reduced pressure to obtain 159.9 g, the content was 99.6%, and the yield was 97%. %, colorless liquid,
[0058] The alkali of step 2) is changed into sodium methylate; Alkyl formate is changed into: ethyl formate;
[0059] 600ml of toluene, 216g (2.0mol, 50%) sodium methoxide, 82.7g (0.5mol, 99.3%) of methyl o-toluene acetate were put in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com