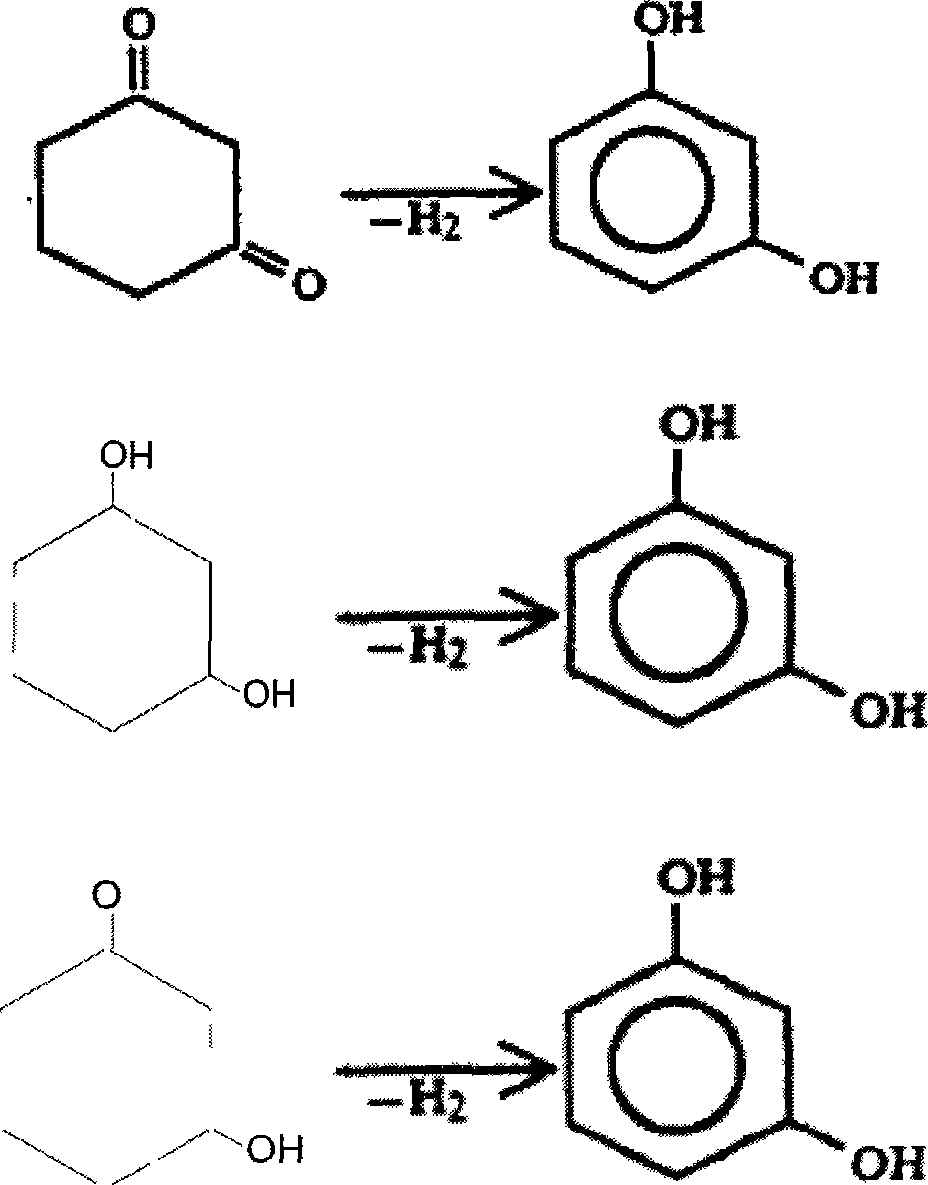
Resorcin synthetic process
A technology for resorcinol and synthesis process, which is applied in the field of synthesis technology of resorcinol, can solve the problems of relatively high requirements on process conditions, high cost, and a conversion rate of only 26%, and achieves guaranteed catalytic effect and low cost. , the effect of reducing catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 1 liter of water and 10 grams of 5% Pd-C catalyst (which contains 8% potassium oxide promoter) in a 5-liter autoclave. After sealing, heat it to 190 ° C under stirring, and feed nitrogen or inert gas under the liquid surface. Keep the liquid surface pressure at about 1.2Mpa. 2 liters of aqueous solution having 180 grams of 1,3-cyclohexanedione dissolved therein was introduced into the reaction kettle within 2 hours from the liquid surface, the temperature was controlled at 180-185° C., and the reaction was continued for 6 hours. After the reaction is finished, cool down to normal temperature, filter and recover the catalyst, extract the reaction solution with butyl acetate to obtain crude resorcinol, distill the crude product resorcinol to recover butyl acetate, and then distill under reduced pressure to obtain a white product with a purity of 99.4%. Resorcinol 163.8 grams. The yield of resorcinol in this embodiment is 92.1%.
Embodiment 2
[0023] In a 5-liter autoclave, add 1 liter of water and 10 grams of 10% Pt-C catalyst (which contains a promoter of 6% magnesium oxide), after sealing, heat to 170 ° C under stirring, and feed nitrogen or inert gas under the liquid surface Keep the liquid surface pressure at about 1.0Mpa. 2 liters of aqueous solution containing 225 grams of 1,3-cyclohexanedione was introduced into the reaction kettle from under the liquid surface within 90 minutes, the temperature was controlled at 170-175° C., and the reaction was continued for 4 hours. After the reaction is finished, lower the temperature to normal temperature, filter and recover the catalyst, extract the reaction solution with isopropyl ether to obtain crude resorcinol, distill the crude product resorcinol to recover isopropyl ether, and then distill under reduced pressure to obtain a white product with a purity of 99.7%. Resorcinol 200 grams. The yield of resorcinol in this embodiment is 90.2%.
Embodiment 3
[0025] In a 5-liter autoclave, add 1 liter of water and 10 grams of 30% Ni-diatomite catalyst (promoter comprising 4% potassium sulfate), after sealing, heat to 180°C under stirring, and feed nitrogen or gas under the liquid surface The inert gas keeps the liquid surface pressure at about 1.4Mpa. 2 liters of aqueous solution having 150 grams of 1,3-cyclohexanedione dissolved in it was introduced into the reaction kettle within 2 hours from the liquid surface, the temperature was controlled at 180-185°C, and the reaction was continued for 4.5 hours. After the reaction is finished, cool down to normal temperature, filter and recover the catalyst, extract the reaction solution with a mixed solvent of butyl acetate and n-butanol to obtain crude resorcinol, distill the crude product resorcinol to recover the solvent, and then distill under reduced pressure to obtain a purity of 99.8 % white resorcinol 139.3 g. The yield of resorcinol in this embodiment is 94.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com