Chemical luminescence immune assay determination reagent kit for rubella virus IgG antibody and preparation method thereof
A chemiluminescence immunity and chemiluminescence technology, which is applied in the field of immunoanalysis medicine, can solve the problems of being unable to be widely used in clinical diagnosis and scientific research work, and restricting the use of popularization, so as to reduce non-specific reactions, improve specificity and sensitivity, and dilute The effect of scaling down
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
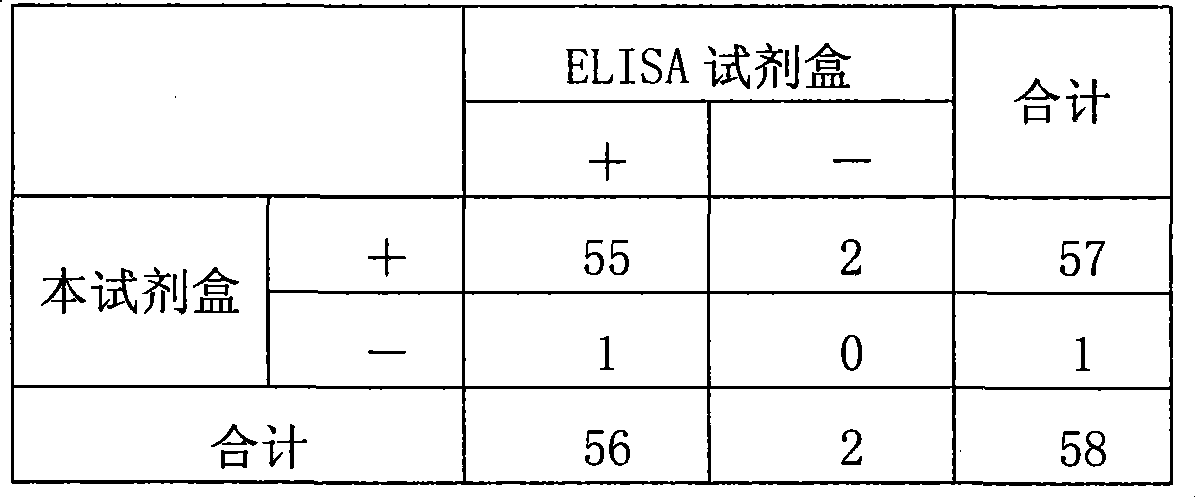
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of rubella virus IgG antibody chemiluminescent immunoassay assay kit of the present invention
[0024] The rubella virus IgG antibody diagnostic kit of the present invention comprises: negative and positive controls, coated rubella virus antigen solid-phase carrier, sample diluent, concentrated washing solution, alkaline phosphatase-labeled anti-human IgG monoclonal antibody, chemiluminescent substrate thing.
[0025] The inventors of the present invention firstly carried out the screening test and quality identification of the raw materials used, including the adsorption performance and variation size of the coated antigen, labeled antibody, white microwell plate, ALP activity, luminescence intensity and luminescence intensity of the chemiluminescence substrate. duration etc. Then test the solid phase carrier with different coating buffers and blocking solutions, select the most suitable coating buffer and blocking solution, and find the best con...
Embodiment 2~3
[0073] Embodiment 2~3 prepare rubella virus IgG antibody chemiluminescence immunoassay assay kit of the present invention
[0074] The assay kit of the present invention was prepared in the same manner as in Example 1 except that plastic beads and plastic tubes were used as carriers respectively.
Embodiment 4
[0075] Embodiment 4 The using method of kit of the present invention
[0076] 1) Take out the kit from the refrigerator at 4°C, and equilibrate at room temperature for 15 minutes.
[0077] 2) After diluting the 20-fold concentrated washing solution provided in the kit with deionized water 20 times, it is prepared as an application washing solution.
[0078] 3) Take out the coated slats and insert them into the plate holder.
[0079] 4) Set 3 wells for negative control and 2 wells for positive control, and add 100uL to each well. Add 100uL of the sample diluent to the well, then add 10uL of the serum to be tested into the sample diluent, and set a blank well for each experiment. Shake and mix well with a micro shaker, paste the sealing film, and incubate at 37°C for 30 minutes;
[0080] 5) Wash the plate 5 times with a fully automatic plate washer or manually, 400uL per well, and finally dry it on clean absorbent paper;
[0081] 6) Except for blank wells, add 100uL of enzym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com