Preparation method of hepatitis b human immunoglobulin for intravenous injection
A technology of immunoglobulin and hepatitis B, which is applied in the direction of digestive system, antiviral agents, drug combinations, etc., can solve the problems of relapse, reduce hepatitis B, and cannot be used in large doses for liver transplant patients, so as to prevent and treat recurrence Effect of recurrence, quality index improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
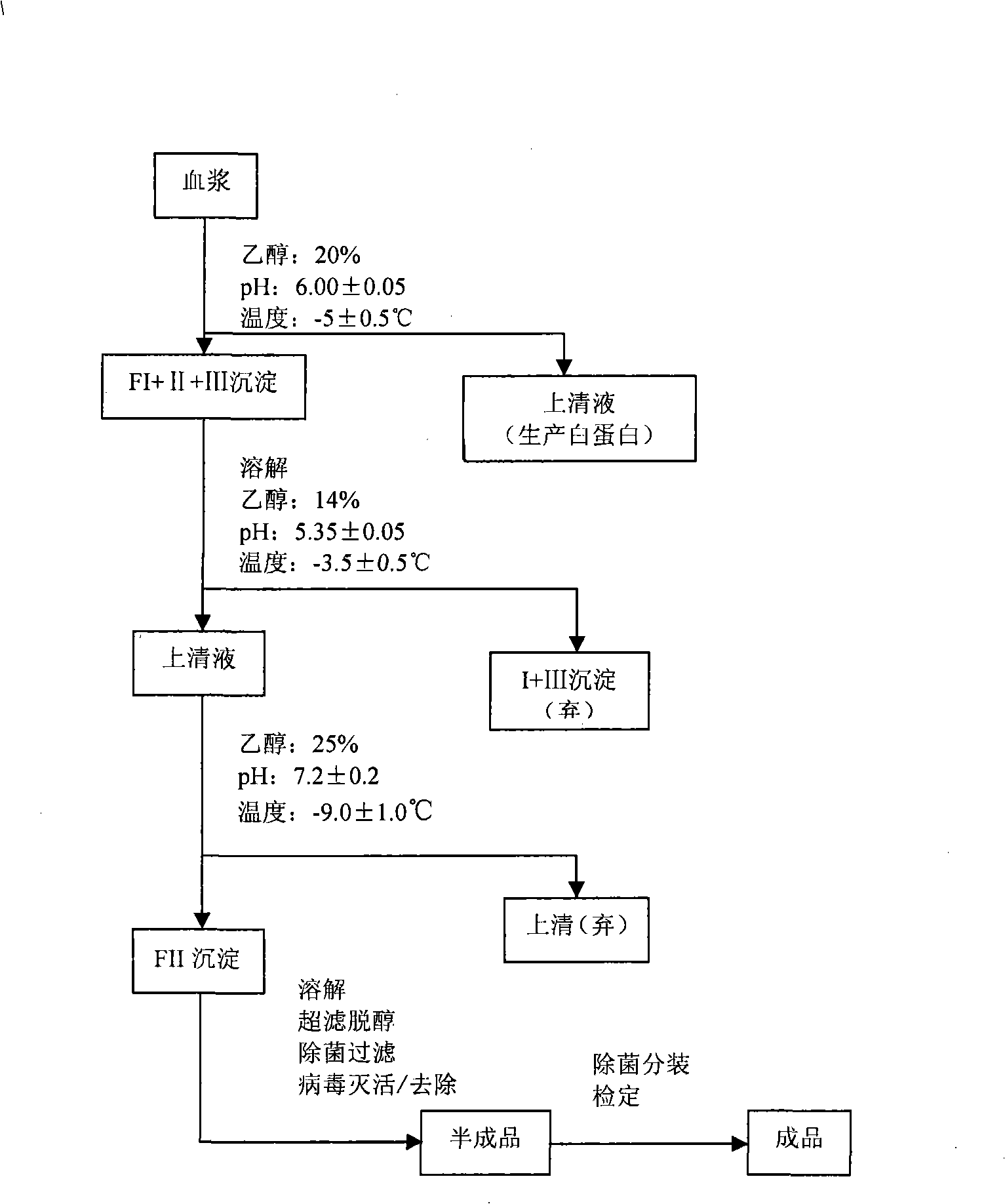
[0023] refer to figure 1 , the preparation method concrete steps of human hepatitis B immunoglobulin (pH4) for intravenous injection of the present invention are as follows:
[0024] 1) For the combined plasma, adjust the protein concentration to 40-50g / L with physiological saline, adjust the final pH of the suspension to 6.00±0.05 with pH4.00 acetate buffer, and adjust with 95% ethanol below -20°C The concentration of ethanol in the solution is 20%, adjust the reaction temperature to -5.0±0.5°C, continue to stir and react for 2 hours, let it stand overnight, and centrifuge to collect the precipitate to obtain FI+II+III (ie component I+II+III);
[0025] Among them, pH4.00 acetate buffer contains 108.08g of sodium acetate and 228.8ml of anhydrous acetic acid per liter of buffer;
[0026] Calculation of ethanol amount: V 95%乙醇 =0.267×V 悬液 (L)
[0027] m 95%乙醇 =0.83×V 95%乙醇 (Kg).
[0028] 2) Completely stir and dissolve the FI+II+III precipitate with 0-2°C cold...
Embodiment 1
[0046] 1) Combine 285L of plasma, add 64L of 0.9% NaCl, adjust the pH of the solution to 5.88 with pH4.00 acetate buffer, adjust the final concentration of ethanol to 20% with 94L of 95% ethanol at -22°C, and retest the final pH of the solution 6.00, the final solution temperature was -4.5°C, stirred for 2 hours, allowed to stand overnight, and centrifuged to obtain 18.3kg of FI+II+III precipitate.
[0047] 2) Completely dissolve 18.3kg of FI+II+III precipitate with 230L of water for injection at 0.5°C, adjust the pH of the solution to 4.85 with the above buffer I, add 215L of water for injection, stir for 1 hour, and adjust the solution with the above buffer II The pH is 5.30, add 220L of cold water for injection, the conductivity is 1.05ms / cm, -21.5°C, 120.2L of 95% ethanol, the final concentration of ethanol is adjusted to 14%, the final pH is 5.35, the temperature is -3.5°C, and stirred for 2 hours Let stand overnight, and centrifuge to obtain a supernatant with a volume o...
Embodiment 2
[0056] 1) Combine 337.1L of plasma, add 81.5L of 0.9% NaCl, adjust the pH of the solution to 5.85 with pH4.00 acetate buffer, adjust the final concentration of ethanol to 20% with 112.8L of 95% ethanol at -22°C, and repeat the test The final pH of the solution was 6.05, the final solution temperature was -5.0°C, stirred for 2 hours, allowed to stand overnight, and centrifuged to obtain 23 kg of FI+II+III precipitate.
[0057] 2) Completely dissolve 23kg of FI+II+III precipitate with 300L of water for injection at 0.5°C, adjust the pH of the solution to 4.80 with the above buffer I, add 300L of water for injection, stir for 1 hour, and adjust the pH of the solution with the above buffer II To 5.25, add 221L of cold water for injection, conductivity of 1.15ms / cm, -21°C, 145.3L of 95% ethanol to adjust the final concentration of ethanol in the solution to 14%, the final pH is 5.30, temperature -4.0°C, and stir for 2 hours. Place overnight, centrifuge, and the volume of the supern...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductance | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com