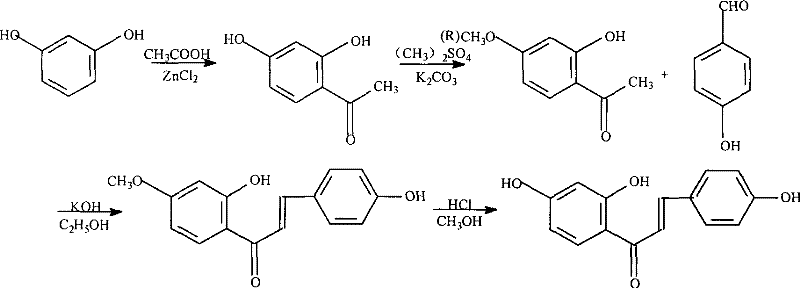
A kind of synthetic method of isoliquiritigenin
A technology of isoliquiritigenin and synthesis method, applied in the direction of condensation preparation of carbonyl compounds, separation/purification of carbonyl compounds, organic chemistry, etc., to achieve the effects of high yield, simple method and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: A. Synthesis of 2,4-dihydroxyacetophenone catalyzed by Lewis base: In a 100ml round bottom flask, add 18g of fully dried resorcinol (placed at 120°C for 30 hours) and 55ml of glacial acetic acid , 20g of molten sodium ethoxide, reflux for 1.5 hours under stirring. After the reactant was cooled to room temperature (25°C, the same below), it was poured into ice water with constant stirring to precipitate crystals, filtered, washed with water 3 times, dried, and recrystallized from ethanol to obtain a yellow solid, namely 15g of 2,4-dihydroxyacetophenone . mp143~144℃ (reference 142~144℃).
[0038] B. Synthesis of 2-hydroxy-4-methoxyacetophenone: add 6.5g of 2,4-dihydroxyacetophenone, 60g of potassium carbonate and 250ml of acetone into a 500ml round bottom flask, and use 50ml for rapid drip at room temperature Me diluted in acetone 2 SO 4 0.03mol, after addition, continue to react for 3 hours, and filter out potassium carbonate. The filtrate was evaporated to dr...
Embodiment 2
[0042] Example 2: A. Synthesis of 2,4-dihydroxyacetophenone under Lewis base catalysis: In a 100ml round-bottomed flask, add 11g of fully dried (placed at 120°C for 24 hours) resorcinol and 30ml of glacial acetic acid , 15g molten ZnCl 2 Reflux for 1 hour under stirring. After the reactant was cooled to room temperature (25°C, the same below), it was poured into ice water with constant stirring to precipitate crystals, filtered, washed with water 5 times, dried, and recrystallized with 50% ethanol to obtain 9g of yellow solid, which is 2,4-dihydroxyl Acetophenone. mp142~144℃ (reference 142~144℃).
[0043] B. Synthesis of 2-hydroxy-4-methoxyacetophenone: Add 4g of 2,4-dihydroxyacetophenone, 44.5g of potassium carbonate and 200ml of acetone to a 500ml round bottom flask, and use 35ml for rapid drip at room temperature 6ml of chloromethyl methyl ether diluted with acetone, after the addition, continue to react for 2 hours, and filter out potassium carbonate. The filtrate was evap...
Embodiment 3
[0047] Example 3: A. Synthesis of 2,4-dihydroxyacetophenone catalyzed by Lewis base: 15g of fully dried resorcinol (placed at 120°C for 24 hours) and 50ml of glacial acetic acid were added to a 150ml round bottom flask , 10g of molten NaH, reflux for 1.5 hours under stirring. After the reactant was cooled to room temperature (25°C, the same below), it was poured into ice water with constant stirring to precipitate crystals, filtered, washed 4 times with water, dried, and recrystallized with 50% ethanol to obtain a yellow solid, namely 2,4-dihydroxystyrene Ketone 12.5g. mp143~144℃ (reference 142~144℃).
[0048] B. Synthesis of 2-hydroxy-4-methoxyacetophenone: add 5.0g of 2,4-dihydroxyacetophenone, 55g of potassium carbonate and 250ml of acetone to a 500ml round bottom flask, and use 40ml for rapid drip at room temperature 8ml of chloromethyl methyl ether diluted with acetone, continue to react for 2 hours after the addition is complete, and filter out potassium carbonate. The f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com