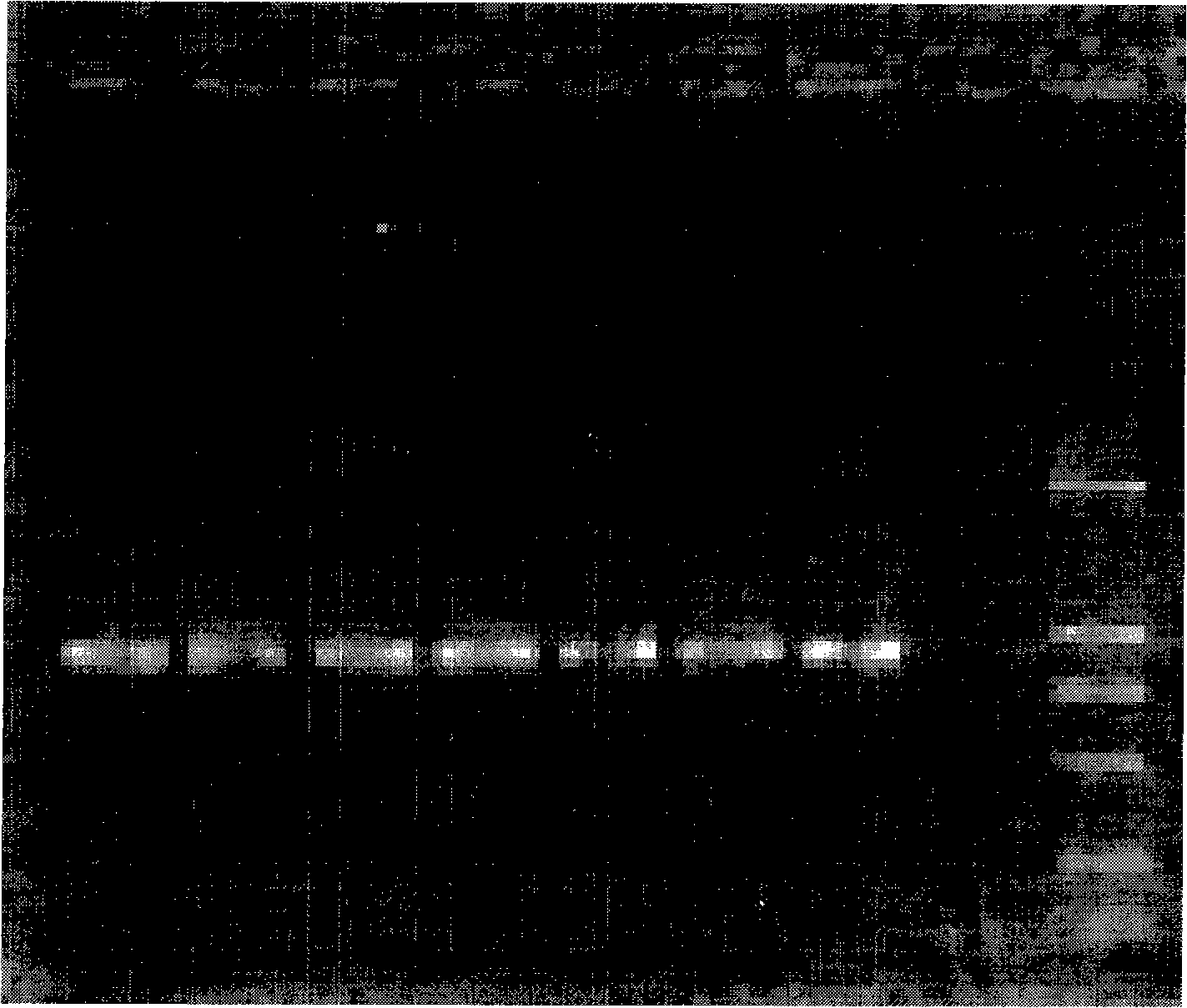Molecular detection method for two Tilletia foetida similar species on bentgrass
A technology for smut and smut, applied in the field of detection of Tilletia graminosa species, can solve the problems of time-consuming and labor-intensive germination experiments, unfavorable rapid detection of ports, etc., to save reagents and time, fast detection, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the germination of experimental material teliospore and the cultivation of mycelium
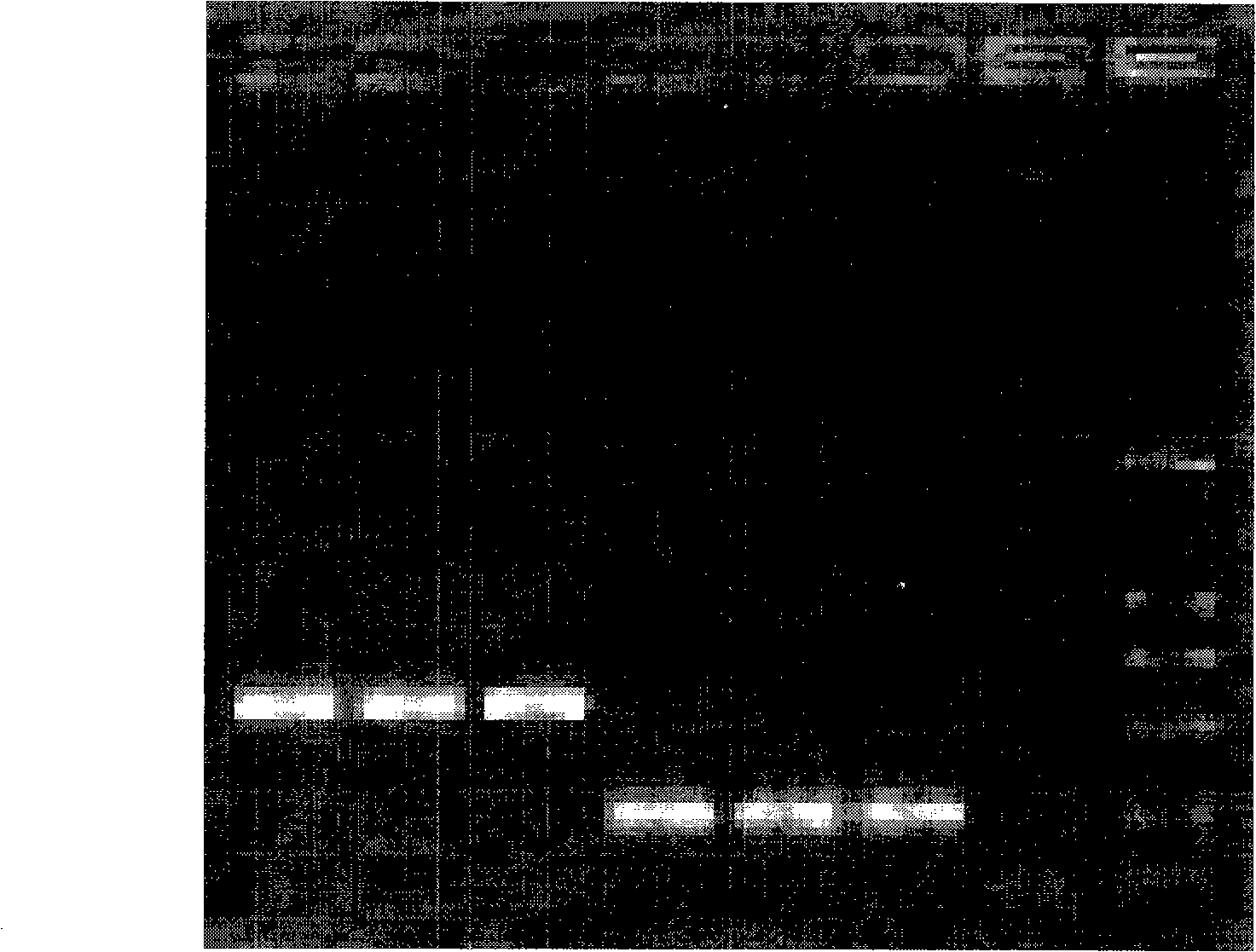
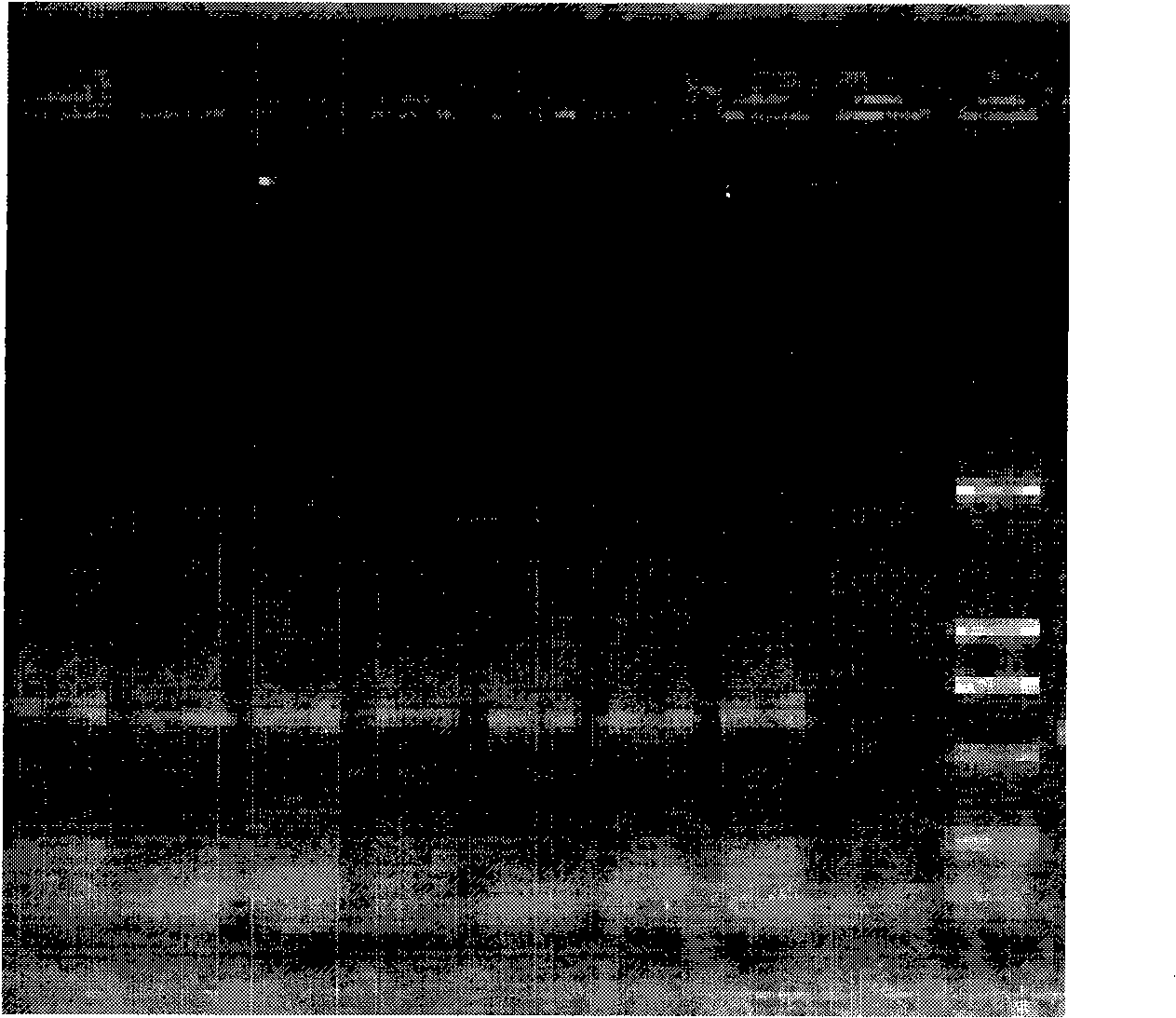
[0042] The materials involved in the experiment include 2 similar species of Tilletia, a total of 11 strains (mycelia and teliospores) from the Phytosanitary Laboratory of the Animal, Plant and Food Testing Center of Tianjin Entry-Exit Inspection and Quarantine Bureau. LMC360 is the specimen for exchange, and LMC360 , TCT3, TCT4, Ts1, Ts3, Ts4 use the cultured mycelium, LMC360, TCT1, TCT2, Ts1, Ts3, Ts5 as the teliospore material in the nested double PCR detection method, relevant information is shown in Table 1.
[0043] Table 1 Test materials
[0044]
[0045] Add an appropriate amount of 0.25% sodium hypochlorite solution to a 0.5mL Eppendorf centrifuge tube, pick a certain amount of teliospores and put them in, mix them with a vortex shaker, sterilize for 50 seconds, centrifuge at 3000 rpm for 1 minute, and immediately add Aspirate the supernatant from the sampler...
Embodiment 2
[0046] Embodiment 2: the extraction of hyphal genome DNA;
[0047] Mycelial DNA was extracted using Shanghai Sangon Genomic DNA Purification Kit (No. SK1252). The extracted genomic DNA was dissolved in 70 μL 1×TE, and the remaining hyphae were stored at -70°C for use.
Embodiment 3
[0048] Embodiment 3: the picking and breaking of teliospores
[0049] Place a 1mm square cover glass on the glass slide, drop about 0.5 μL of 10×PCR buffer (10mmol / L Tris-HCl, 50mmol / L KCl, 1.5mmol / L MgCl 2 , pH8.3), puncture the gall with a dissecting needle, pick about 3-10 teliospores and place them in 10×PCR buffer, cover with a cover glass of similar size, rub the cover glass gently with tweezers, and examine the After confirming the rupture of the spores, put the superimposed two coverslips together into the bottom of the PCR tube containing 4.5 μL 10×PCR buffer, cover the tube cap, and use no teliospores but only the PCR buffer as a negative control.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com