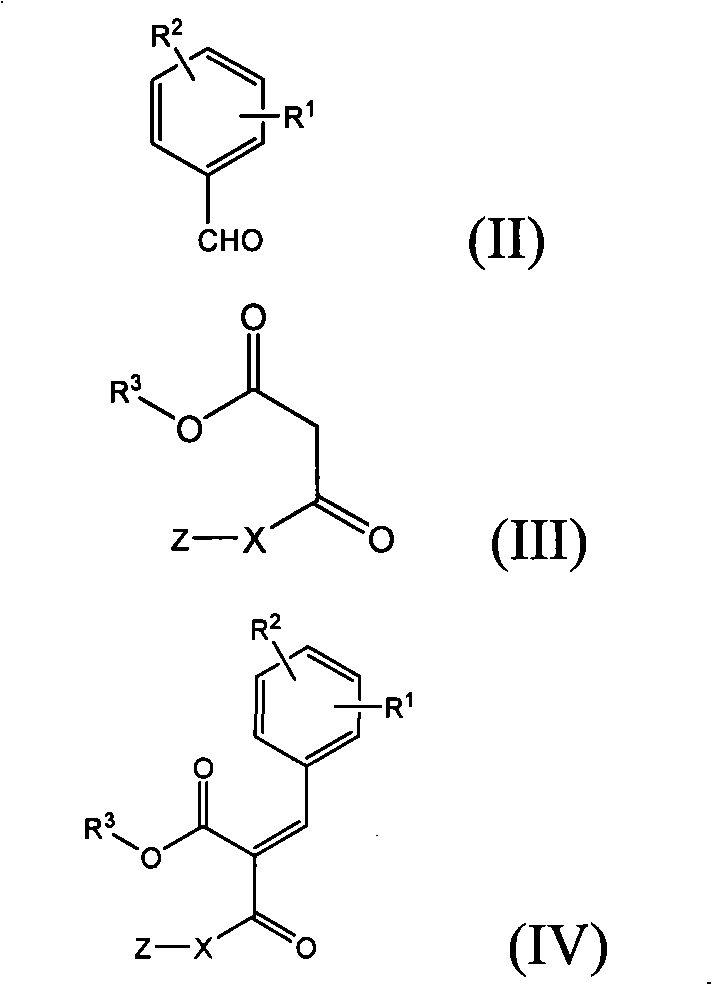
Diethylcarbamyl-substituted thiazole dihydropyrimidine
A thiazole and alkyl technology, applied in the field of ethoxycarbonyl-substituted thiazole dihydropyrimidines, can solve problems such as side reactions and rebound effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Ethyl 4-(2-chloro-4-fluorophenyl)-2-(thiazol-2-yl)-6-methyl-1,4-dihydropyrimidine-5-carboxylate
[0134]
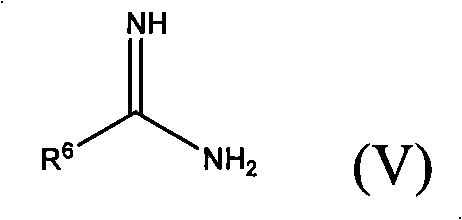
[0135] 10.0g (63.1mmol) of2-chloro-4-fluorobenzaldehyde, 8.2g (63.1mmol) ethylacetoacetic acid, 10.3g (63.1mmol) 2-amidino-thiazole hydrochloride and 6.2g (75.7mmol) acetic acid Sodium was dissolved or suspended in 500ml of ethanol and boiled under reflux for 16 hours. Cool to room temperature, filter with suction, and wash the residue with water to remove inorganic salts. 12.8 g (53.4%) of product were obtained, melting point: 162-164°C.
Embodiment 2
[0137] Methyl 4-(2-chloro-4fluorophenyl)-2-(thiazol-2-yl)-6-methyl-1,4-dihydropyrimidine-5-carboxylate This compound uses methyl acetoacetate Synthesized according to the method similar to Example 1.
[0138] Yield: 55% (melting point: 152-154°C)
Embodiment 3
[0140] Ethyl 4-(2-bromo-4fluorophenyl)-2-(thiazol-2-yl)-6-methyl-1,4-dihydropyrimidine-5-carboxylate This compound uses ethyl acetoacetate Synthesized according to the method similar to Example 1.
[0141] Yield: 51.6% (melting point: 163-165°C)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com