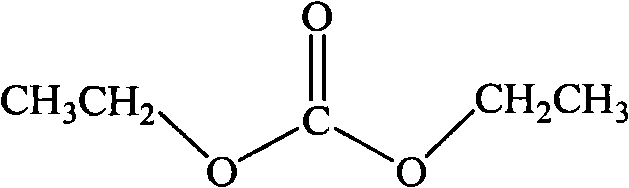
Method for preparing diethyl carbonate by two-step coupling reaction
A technology of diethyl carbonate and coupling reaction, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carbonate/haloformate, etc., can solve the problem of low yield of urea alcoholysis method and expensive raw materials of transesterification method Economic and other issues, to achieve the effect of convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
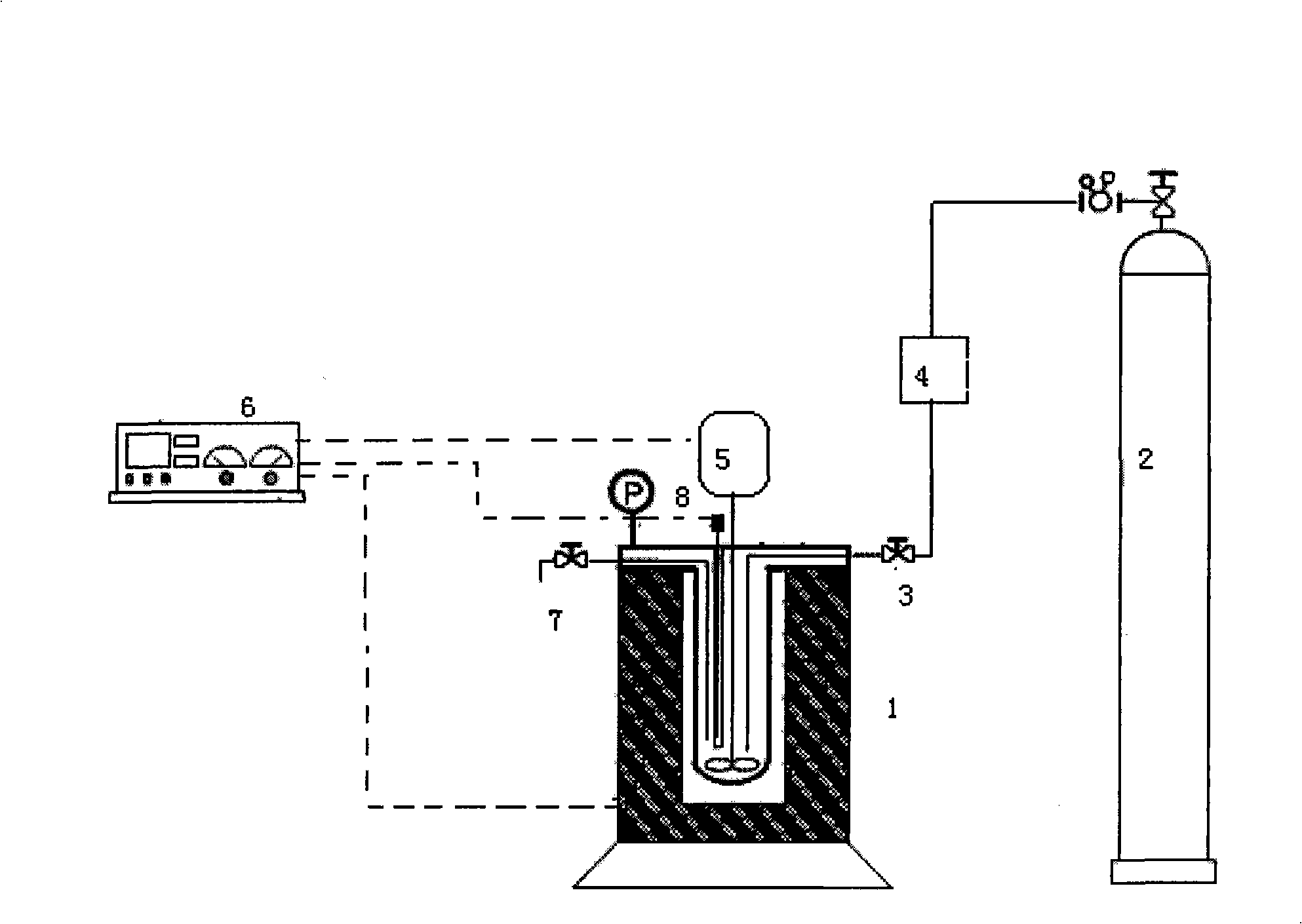
[0024] 0.25 moles of urea, 0.75 moles of ethanol, 2.5 moles of ethylene glycol, and 20.45 grams of zinc oxide catalyst were added to the reactor 1, and the nitrogen pressure was filled to 0.6 MPa. After heating the mixed solution to 170°C, turn on the agitator 5, start the reaction by timing, and control the pressure in the reactor to 2.2Mpa. After the reaction was completed, the reactor was cooled to room temperature, and gas chromatography was used to analyze the composition of the mixture in the reactor to determine the yield of the product. After 5 hours of reaction, the yield of diethyl carbonate reached a maximum of 78%.
Embodiment 2
[0026] Add 0.25 moles of urea, 0.75 moles of ethanol, 1.5 moles of ethylene glycol, and 22.75 grams of zinc oxide catalyst into reactor 1. The initial nitrogen pressure is 0.7 MPa, the reaction temperature is 170 ° C, the stirring speed is 700 rpm, and the pressure in the reactor is maintained at 2.5Mpa, reaction 4 hours diethyl carbonate yield is 53.7%, all the other conditions are with embodiment 1.
Embodiment 3
[0028] Add 0.25 moles of urea, 1.75 moles of ethanol, 2.1 moles of ethylene glycol, and 20.95 g of zirconia catalyst into the reaction kettle, and the initial nitrogen pressure is 0.8 MPa. After heating the mixture to 180 ° C, under the action of stirring speed of 600 rpm react. The pressure in the reactor was maintained at 3.0Mpa, and after 4 hours of reaction, the yield of DEC was 49.13%, and the other conditions were the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com