Losartan potassium and hydrochlorothiazide tablets and preparation method thereof
A technology of losartan potassium hydrochlorothiazide tablets and losartan potassium, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Low, long disintegration time and other problems, to achieve the effect of good dissolution rate, good dissolution rate and good stability of the tablet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
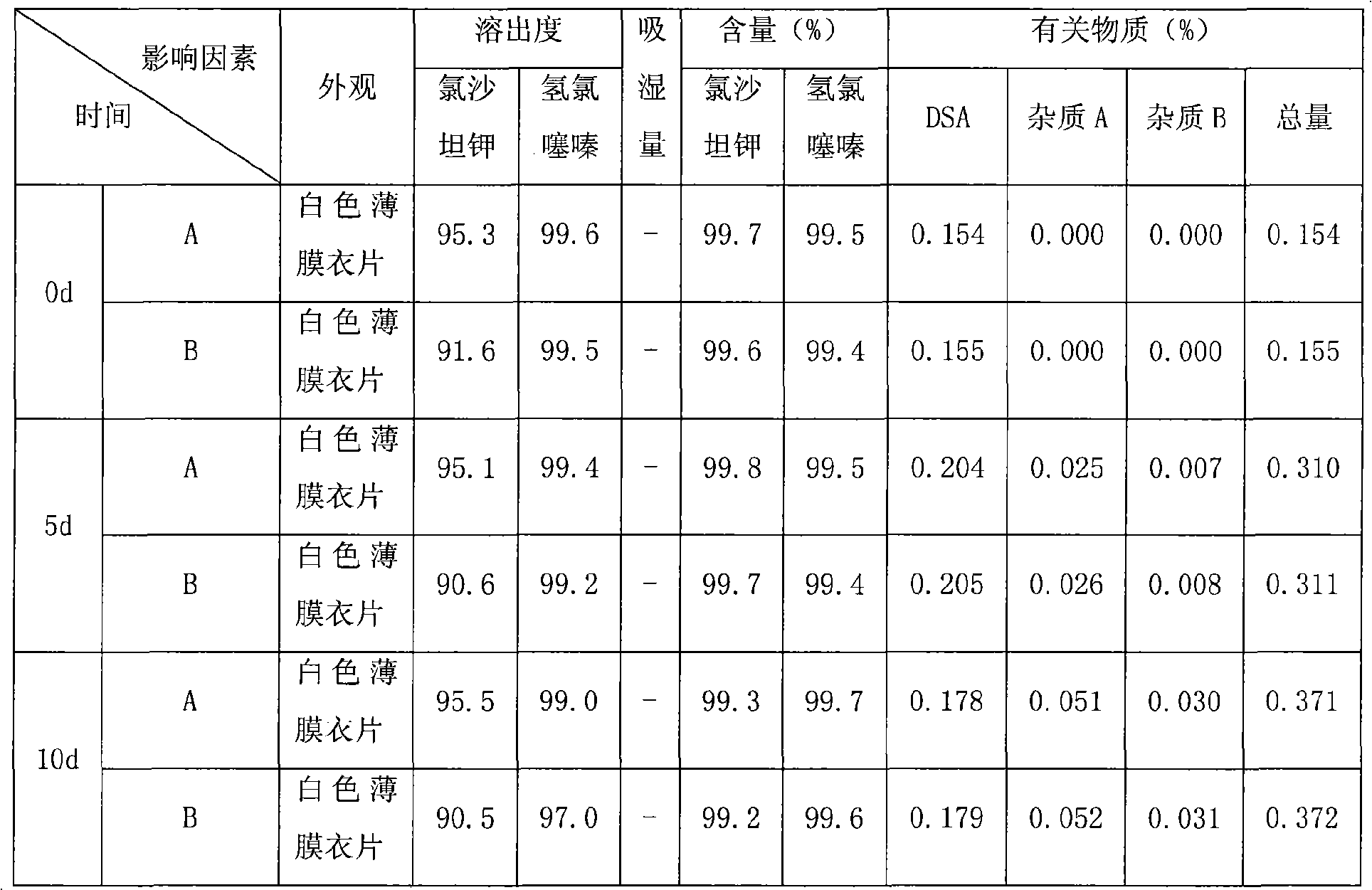
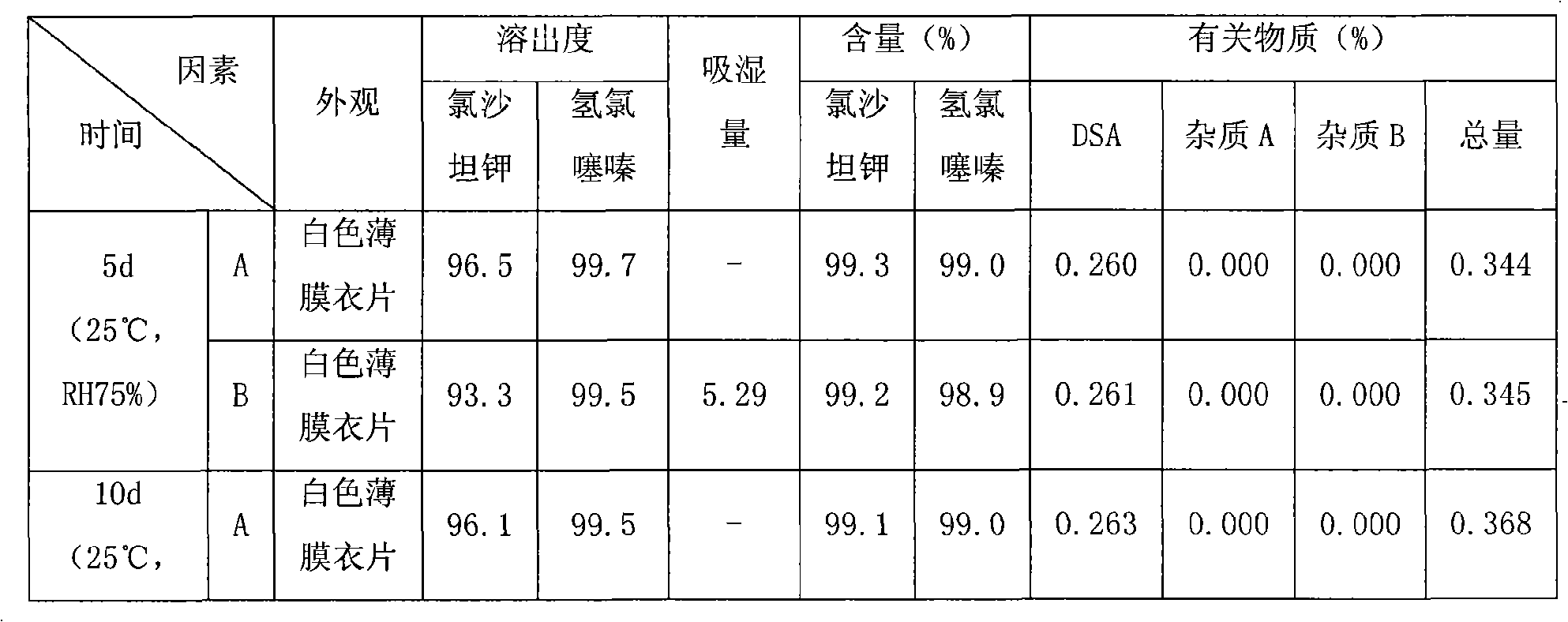
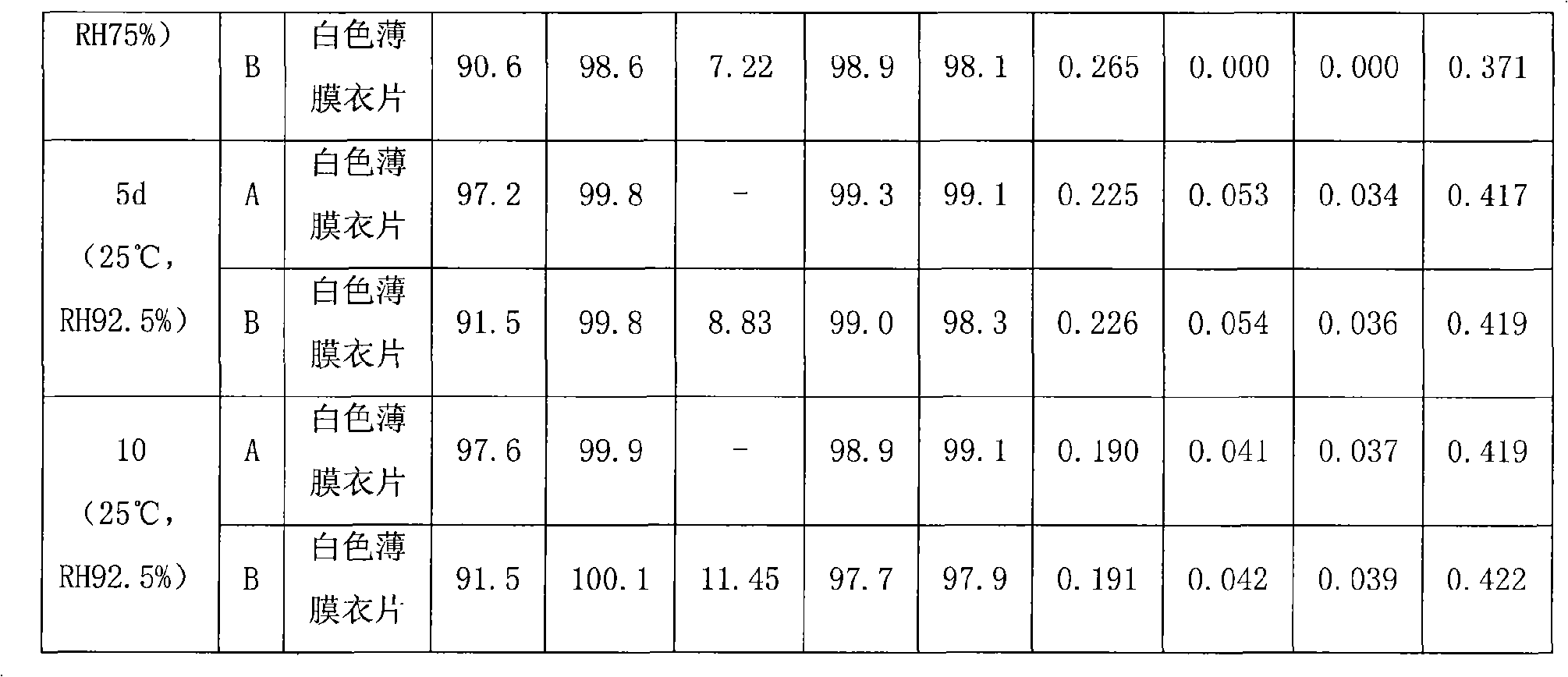
Image
Examples
Embodiment 1
[0056] (1) Prescription:
[0057] Tablet prescription:
[0058] Losartan Potassium 50mg
[0059] Hydrochlorothiazide 12.5mg
[0060] Microcrystalline Cellulose 80mg
[0061] Lactose monohydrate 80mg
[0062] Pregelatinized starch 12mg
[0063] Povidone K30 2.4mg
[0064] Magnesium Stearate 2.4mg
[0065]
[0066] Total 239.3mg
[0067] Coating Solution Prescription:
[0068] 7.2mg
[0069] Purified water 36mg
[0070] (2) Preparation method:
[0071] (a) mixing losartan potassium, hydrochlorothiazide, and lactose monohydrate;
[0072] (b) Povidone K30 is dissolved in 75% ethanol;
[0073] (c) Mix the product obtained in step (a) and step (b) uniformly, granulate with a 24-mesh sieve, dry at 60° C., pass the dry granules through a 24-mesh sieve for granulation;
[0074] (d) mixing the product obtained in step (c) with microcrystalline cellulose, pregelatinized starch, and magnesium stearate evenly, measuring the content of th...
Embodiment 2
[0077] (1) Prescription:
[0078] Tablet prescription:
[0079] Losartan Potassium 40mg
[0080] Hydrochlorothiazide 10mg
[0081] Microcrystalline Cellulose 70mg
[0082] Lactose monohydrate 70mg
[0083] Pregelatinized starch 10mg
[0084] Povidone K30 2mg
[0086] Coating Solution Prescription:
[0087] 7.2mg
[0088] Purified water 36mg
[0089] (2) Preparation method:
[0090] (a) mixing losartan potassium, hydrochlorothiazide, and lactose monohydrate;
[0091] (b) Povidone K30 is dissolved in 75% ethanol;
[0092] (c) Mix the product obtained in step (a) and step (b) uniformly, granulate with a 24-mesh sieve, dry at 60° C., pass the dry granules through a 24-mesh sieve for granulation;
[0093] (d) Mix the product obtained in step (c) with microcrystalline cellulose, pregelatinized starch, and magnesium stearate evenly, measure the content of the main ingredient, and press the tablet after determining the weight of...
Embodiment 3
[0095] (1) Prescription:
[0096] Tablet prescription:
[0097] Losartan Potassium 60mg
[0098] Hydrochlorothiazide 15mg
[0099] Microcrystalline Cellulose 90mg
[0100] Lactose monohydrate 90mg
[0101] Pregelatinized Starch 14mg
[0102] Povidone K30 2.8mg
[0103] Magnesium Stearate 2.8mg
[0104] (2) Preparation method:
[0105] (a) mixing losartan potassium, hydrochlorothiazide, and lactose monohydrate;
[0106] (b) Povidone K30 is dissolved in 75% ethanol;
[0107] (c) Mix the product obtained in step (a) and step (b) uniformly, granulate with a 24-mesh sieve, dry at 60° C., pass the dry granules through a 24-mesh sieve for granulation;
[0108] (d) Mix the product obtained in step (c) with microcrystalline cellulose, pregelatinized starch, and magnesium stearate evenly, measure the content of the main ingredient, and press the tablet after determining the weight of the tablet. Compression under the condition of humidity 65%;
[0109] (e) Put the tablet prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com