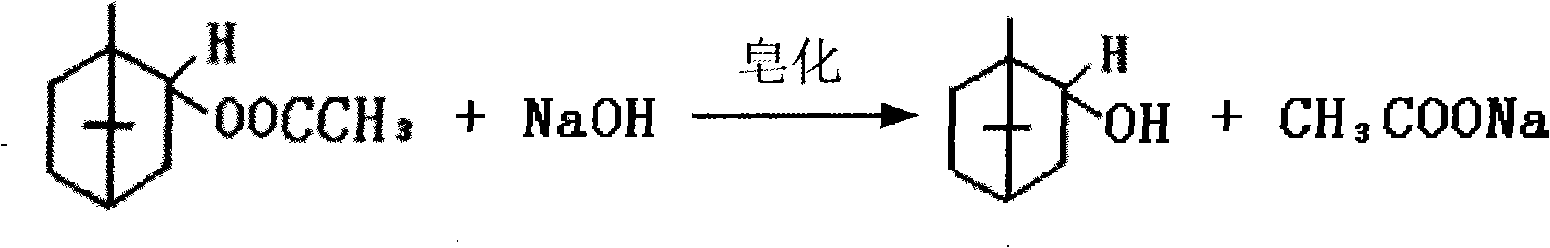
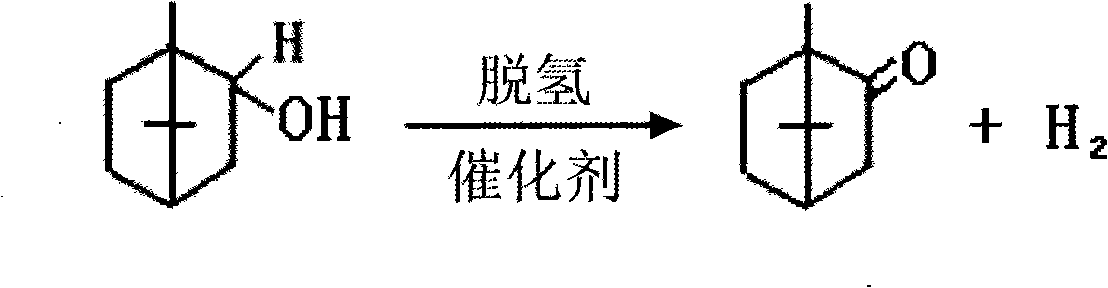
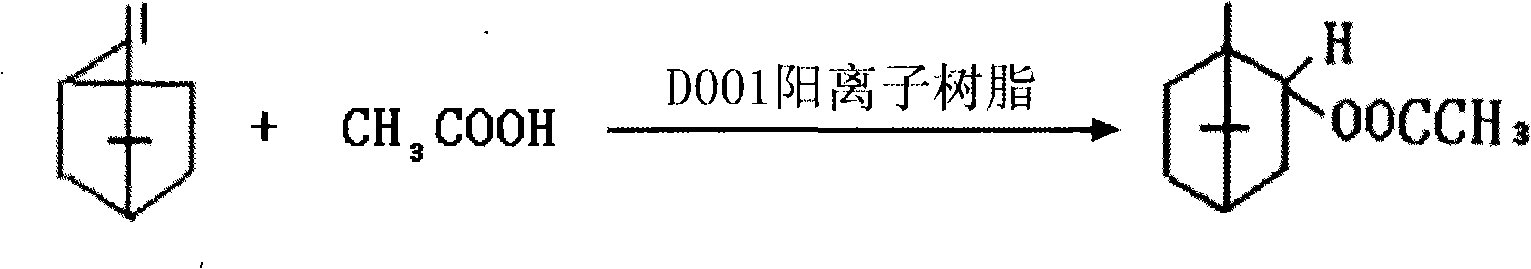Preparation of isobornyl acetate
A technology of isobornyl acetate and acetic acid, which is applied in the field of preparation of isobornyl acetate, can solve the problems of reduced selectivity, reduced quality, and many harmful impurities, so as to reduce the probability of rearrangement reaction, improve selectivity, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 2250kg of industrial camphene, 1050kg of industrial acetic acid, pumped into the reaction kettle equipped with 10% D001 cationic resin, stirred and reacted for 2 hours, controlled temperature at 22~25°C. According to the chromatographic analysis of the reaction, the content of isobornyl acetate was 74.8%, the content of fennel and 3-camphor was 2.9%, the conversion rate of camphene esterification was 72.4%, and the selectivity of isobornyl acetate was 98.1%.
[0022] The esterification reactants are sent to the refined ester-finished ester series distillation tower for distillation, and the vacuum at the top of the tower is 0.093MPa. The refined ester extracted from the refined ester distillation tower contains 94.8% of isobornyl acetate and 1.3% of terpene light components. The temperature at the bottom of the tower is 130°C, and the isobornyl acetate is extracted from the liquid phase side line in the middle of the finished ester distillation tower. The extraction temp...
Embodiment 2
[0024] 720kg of industrial camphene, 230kg of acetic acid, and 2140kg of camphene solution are pumped into a reaction kettle equipped with 10% D001 cationic resin and stirred for 3.5 hours. The temperature is controlled at 20-25°C with low-temperature water, and the reaction solution is analyzed by chromatography for acetic acid. The isobornyl ester content is 69.5%, the fennel ester is 2.6%, the 3-camphoryl ester is 0.65%, the camphene conversion rate is 68.5%, and the isobornyl acetate selectivity is 97.2%.
[0025] The esterification reactants are sent to the refined ester-finished ester series distillation tower for distillation, the vacuum degree at the top of the tower is 0.093MPa, the temperature of the refined ester extracted from the refined ester distillation tower is 130.2°C, the content of isobornyl acetate is 95.3%, and the light components of terpenes 1.7%, the content of isobornyl acetate extracted from the liquid phase side line in the middle of the finished pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com