Methods and compositions for inhibiting HIV infection
A compound, pseudovirus technology, applied in the field of new compounds that inhibit HIV infection, can solve the problems of toxicity or adverse side effects, incompatibility of antiviral activity, unsatisfactory drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1. General Materials and Methods
[0087]Cell Lines and Maintenance: HeLaCD4βgal cells were obtained from Dr. Michael Emerman through the AIDS Research and Reference Reagents Program of the NIH, NIAID AIDS Division (Kimpton, J. Virol. 66:2232-9, 1992). Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum, 1× penicillin / streptomycin / L-glutamine, 0.2 mg / mL G418 and 0.1 mg / mL hygromycin B. Jurkat cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1× penicillin / streptomycin L-glutamine. All cell culture reagents were purchased from Invitrogen.
[0088] Screening of cDNA in HeLaCD4βgal cells: High-throughput cDNA reverse transfection of HeLaCD4βgal cells was performed essentially according to the method described in Chanda et al., Proc. Briefly, individual cDNAs from a subgenome library containing 15,000 genes (see http: / / function.gnf.org for details), the negative control Sport6GFP cDNA, and the positive con...
Embodiment 2
[0094] Example 2. Identification of novel HIV-interacting host factors from siRNA screens
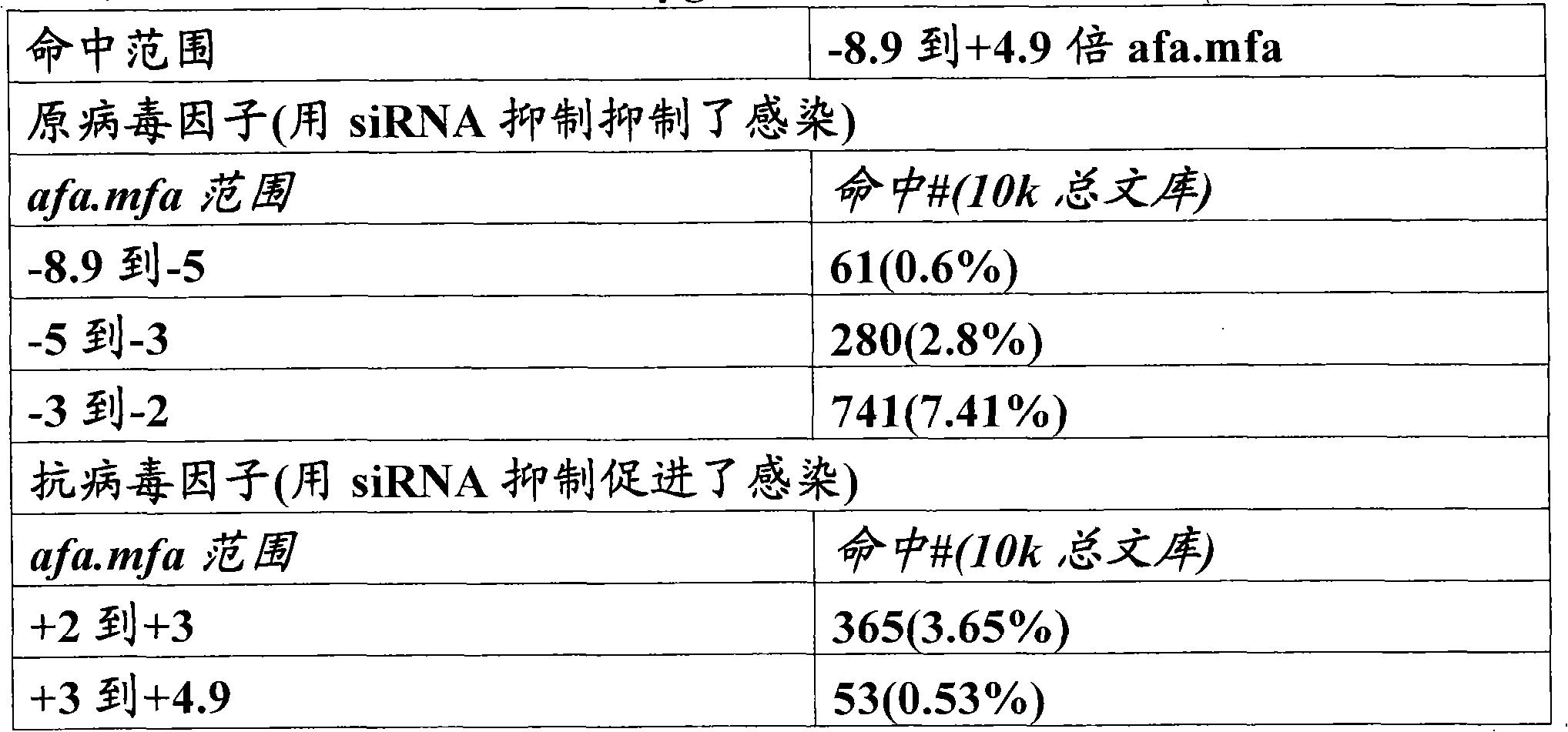
[0095] We performed a subgenomic siRNA screen for host proteins involved in HIV infection by monitoring the expression of a reporter gene under the control of the HIV LTR promoter in HeLaCD4Bgal cells (Kimpton et al., J Virol 66:2232-2239, 1992). HeLa-CD4-Bgal cells were obtained from Dr. Michael Emerman through the AIDS Research and Reference Reagents Program of the NIH, NIAID AIDS Division. Cells were transfected with siRNA against Tat as a positive control using a reverse transfection protocol and challenged with HIV-IIIb 24 hours after transfection (Huang et al., Proc. Natl. Acad. Sci. U.S.A. 101:3456-61 , 2004). In order to be able to observe effects on all stages of infection (from entry, to release, to spread in cell culture), the infection was continued for three days. By monitoring reporter gene expression in HeLaCD4βgal cells, this system can detect any modulation of HIV inf...
Embodiment 3
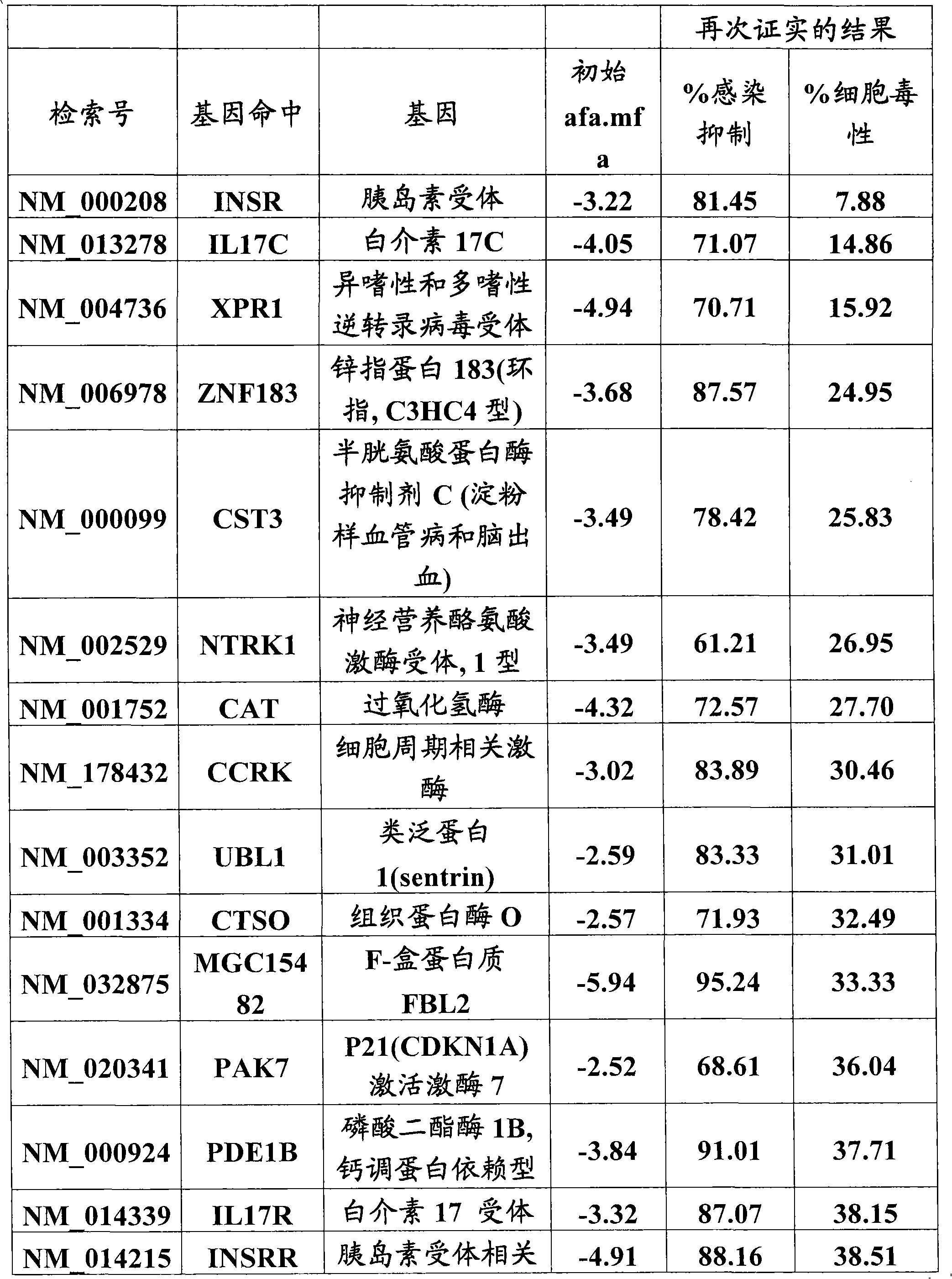
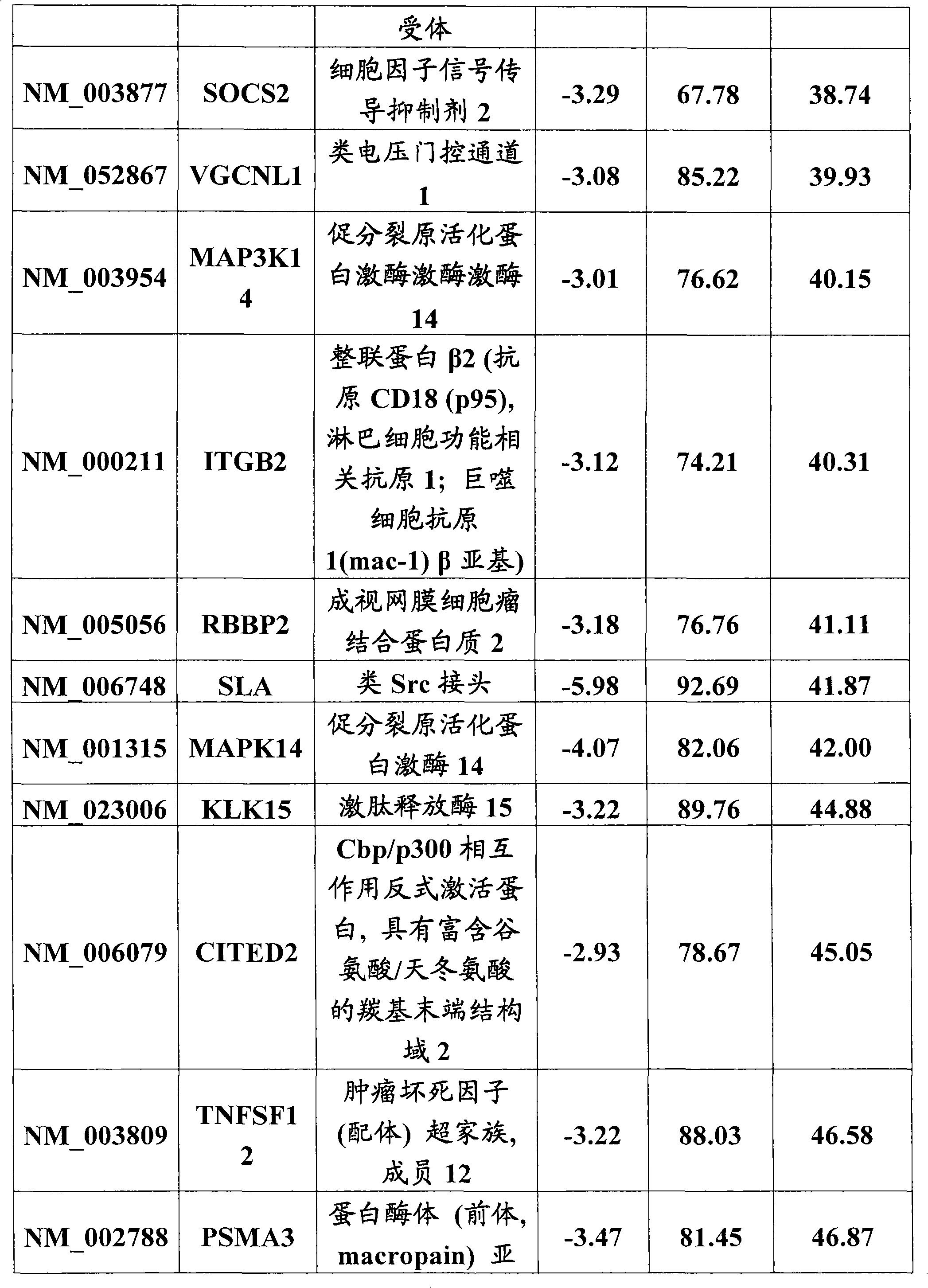
[0097] Example 3 Identification and Characterization of HIV Interacting Host Factors from cDNA Screening
[0098] We performed a high-throughput screen of a cDNA library containing 15,000 unique genes to find novel proviral factors whose overexpression would lead to enhanced HIV-IIIb infection. Since HeLaCD4βgal cells are easy to transfect, we used them for selection and challenge with replication competent HIV-IIIb. Negative control (Sport6-gfp) and positive control (Tat-Sport6) cDNAs were added to the wells of a 384-well plate containing library cDNA (one gene per well), followed by LTR-luciferase reporter gene containing HIVTat response Construct transfection reagent solution. Cells were added after complex formation, and 24 hours later, each well was infected with HIV-IIIb. In order to be able to observe the effect on all stages of the infection (from entry, to release, to spread in the cell culture), the infection was continued for three days. Infection was then assess...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com