Preparation of red fluorescent powder Zn2SiO4:Eu3+
A red fluorescent powder, eu2o3 technology, applied in the direction of chemical instruments and methods, luminescent materials, etc., can solve the problems of inapplicable silicate, urea low reducing ability, low reducing ability, etc., to overcome the reaction activation energy barrier, product The crystal shape becomes better and the effect of increasing the heat release of combustion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
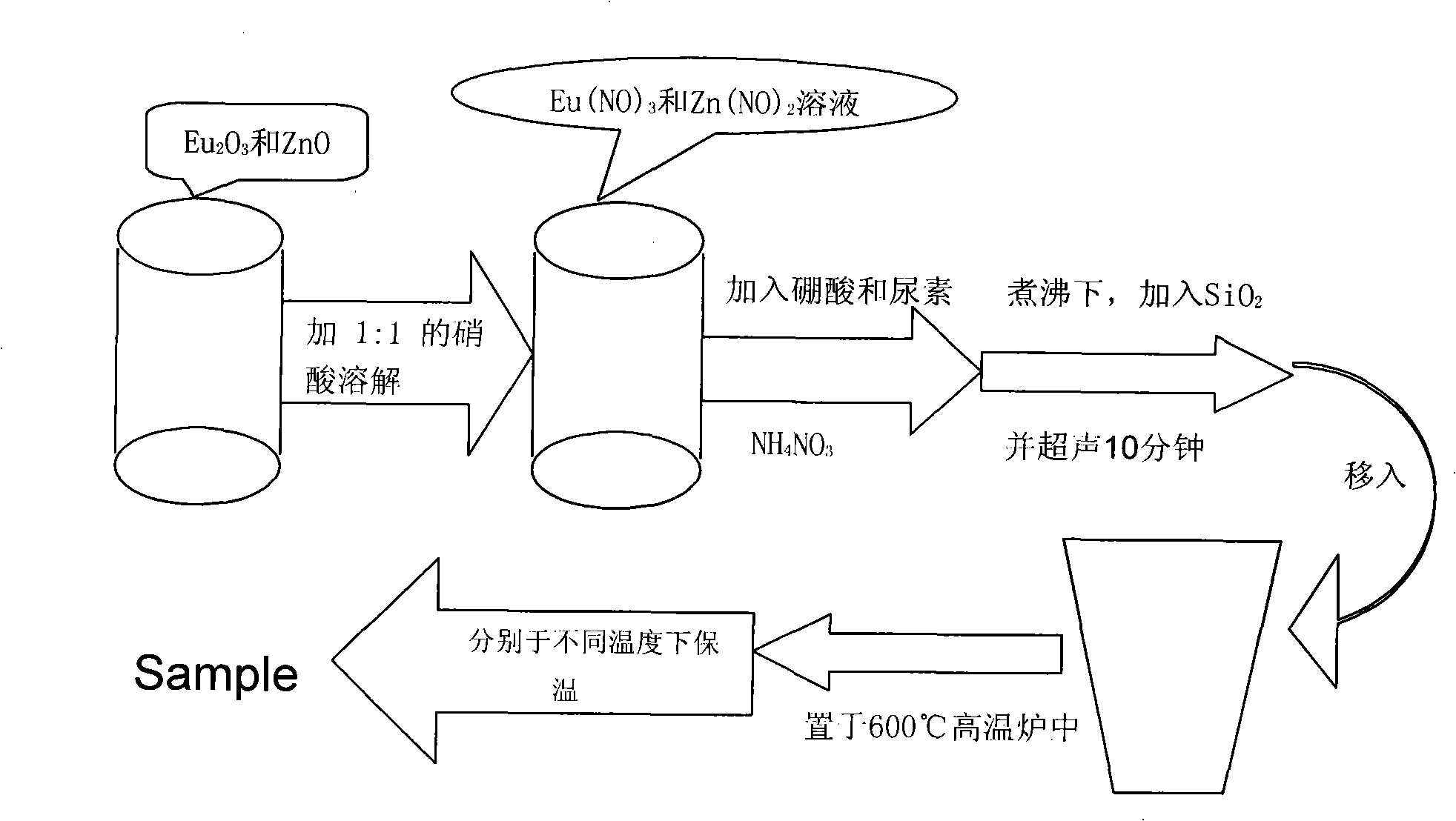
[0027] like figure 1 The process flow diagram shown, a red phosphor Zn 2 SiO 4 :Eu 3+ The preparation method, its steps are as follows:
[0028] 1) the Eu 2 o 3 and ZnO according to the composition ratio (n Eu 2 o 3 : nZnO=0.06:1) dissolved in nitric acid with a volume of 1:1 concentration and mixed evenly;
[0029] 2) Add a certain amount (the content is 2% to 3% of the total mass of other solid raw materials added) of boric acid, urea and combustion aid NH 4N o 3 , in the case of solution boiling SiO 2 (n SiO 2 : nZnO=1:2) was slowly added, and then ultrasonically dispersed for 5-10 minutes;
[0030] 3) Move the solution directly into a high-temperature furnace pre-heated to 600°C. With the evaporation of water, the solution begins to expand and bubble, releasing a large amount of gas; the gas is ignited and burns violently, and the flame is yellow-white. It can be maintained for about 30s;
[0031] 4) Collect the obtained fluffy and foamy products, grind and mi...
Embodiment 1
[0036] According to the composition ratio n(ZnO):n(Eu 2 o 3 )=1:0.06 Weigh ZnO, Eu 2 o 3 Dissolve 0.1mol (8.1380g) and 0.002mol (0.7040g) respectively in nitric acid at a concentration of 1:1 (volume ratio), and mix them uniformly. Add about 0.17-0.18g (0.1768g) of boric acid, 50.7271g of urea and an appropriate amount of combustion aid NH 4 NO 3 , 0.1mol (6.0080g) SiO 2 Add slowly, then ultrasonically disperse for 10min. Move the solution directly into a high-temperature furnace preheated to 600°C. As the water evaporates, the solution begins to expand and bubble, releasing a large amount of gas. After the gas is ignited, it will burn violently, the flame is yellowish white, and the burning process can last for about 30s. Collect the obtained fluffy and foamy products, place them in an agate mortar, grind and mix them, and finally place them in a high-temperature resistance furnace at 1100° C. for 2 hours. The calcined product is slightly ground to obtain a powder sam...
Embodiment 2
[0038] According to the composition ratio n(ZnO):n(Eu 2 o 3 )=1:0.06 Weigh ZnO, Eu 2 o 3 0.1mol (8.1380g) and 0.002mol (0.7040g), respectively, were dissolved in nitric acid at a concentration of 1:1 (volume ratio), and mixed uniformly. Add about 0.17-0.18g (0.1768g) of boric acid, 40.5817g of urea and an appropriate amount of combustion aid NH 4 NO 3 , 0.1mol (6.0080g) SiO 2 Add slowly, then ultrasonically disperse for 10min. Move the solution directly into a high-temperature furnace preheated to 600°C. As the water evaporates, the solution begins to expand and bubble, releasing a large amount of gas. After the gas is ignited, it will burn violently, the flame is yellowish white, and the burning process can last for about 30s. Collect the obtained fluffy and foamy products, place them in an agate mortar, grind and mix well, and finally place them in a high-temperature resistance furnace at 1100° C. for 3 hours. The calcined product is slightly ground to obtain a powde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com