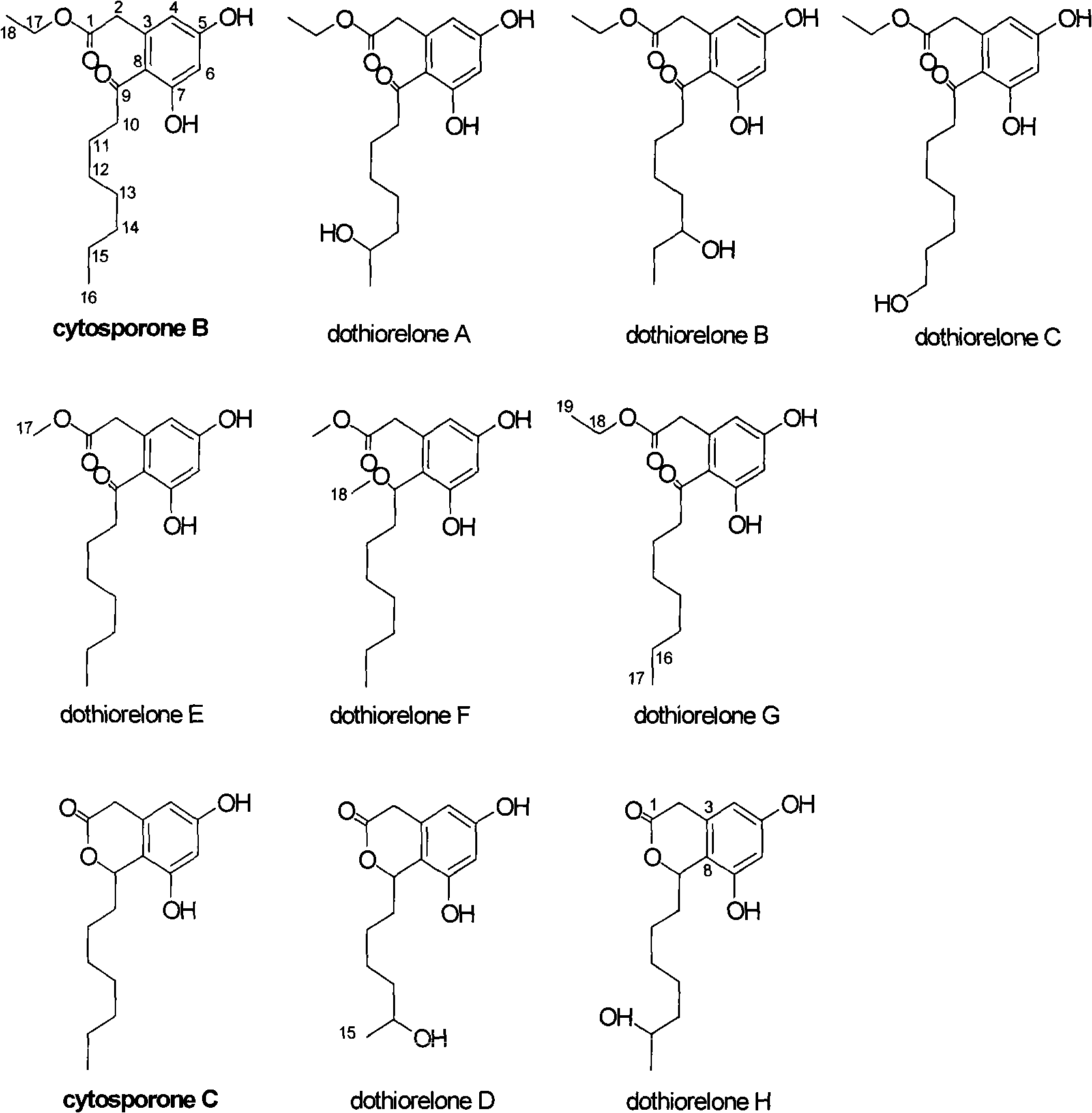
Substituted phenol and application thereof as receptor TR3 excitant
A technology of orphan receptors and phenol derivatives, applied in the field of substituted phenols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
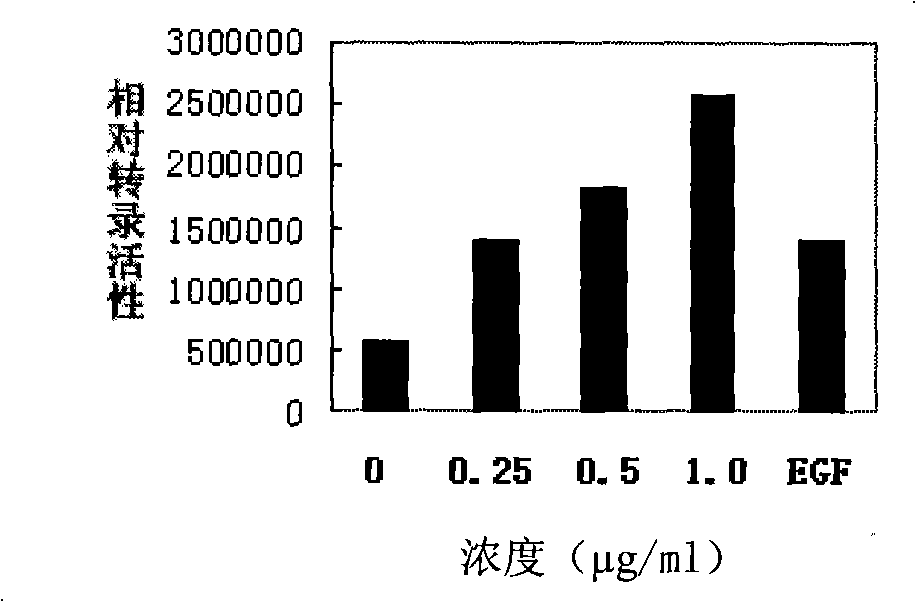
[0032] Example 1, detection of TR3 transcriptional activity
[0033] The reporter gene assay was used to detect the effect of the test compound on inducing the expression of the luciferase reporter gene, so as to reflect the level of TR3 transcriptional activity.
[0034] The test method is as follows:
[0035] Take 5×10 4 Inoculation amount of cells / well Cells were inoculated in a 24-well plate, and the receptor (TR3) expression vector, luciferase reporter gene plasmid (containing Luc-NurRE ) and β-galactosidase expression plasmids were transfected into HEK293T cells. After 24 hours of transfection, fresh complete culture medium was changed, and the treatment group was treated with the compound to be tested at the same time. Discard the culture medium after several hours of treatment, wash the cells twice with PBS, add 120 μl of lysate to each well, place at 4°C for 10 minutes, collect, centrifuge at 13,000 r / min for 5 minutes, and take the supernatant to measure luciferas...
Embodiment 2
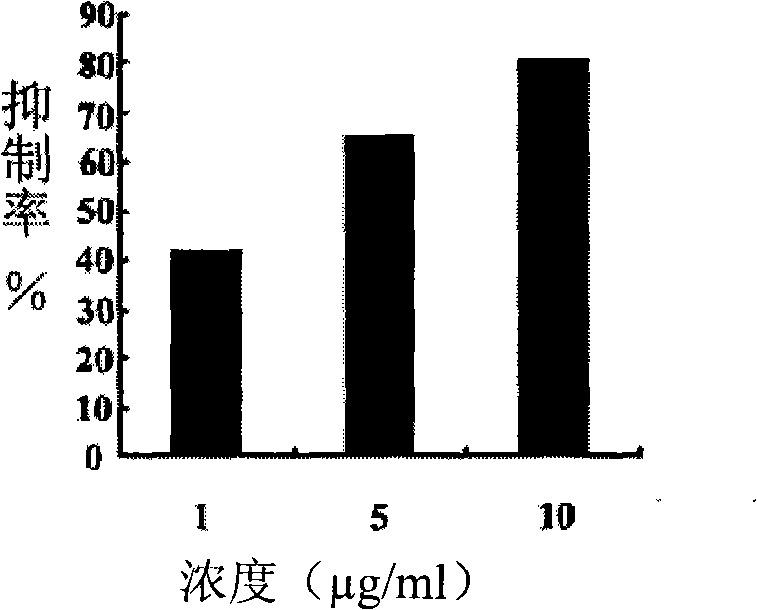
[0039] Embodiment 2, in vitro antitumor activity detection
[0040] MTT (Methyl Thiazolyltetrazolium) method was used to detect the inhibitory effect of compounds on tumor cells in vitro.
[0041] The test method is as follows:
[0042] The cultured tumor cells (human B lymphoma Raji cells, human oral dermoid carcinoma KB cells and human gastric cancer cells BGC-823) were made into single cell suspension, counted by hemocytometer and diluted to a cell concentration of 6×10 4 individual / mL. Inoculate cells into 96-well plates, 80 μL per well. In addition, set 2 cells containing only 80 μL RMPI 1640 complete medium [cell culture medium: a bag of 10g dry powder RMPI 1640 (Gibco Co.Ltd.) cell culture medium was dissolved in 1L double distilled water; add 2g NaHCO 3 Stir well and dissolve, seal, and place at 4°C overnight to remove impurities by natural precipitation; add 10-15% inactivated (56°C, 30min) calf serum and 1% polyclonal antibody mother solution the next day; mix wel...
Embodiment 3
[0049] Embodiment 3, cell apoptosis analysis
[0050] DAPI staining and flow cytometry were used to detect the effect of compounds on inducing tumor cell apoptosis.
[0051] The test method is as follows:
[0052] DAPI-stained cell count: After treating the tumor cells with the compound to be tested (see the above-mentioned Example 1 for the method) for 24 hours, the tumor cells were washed twice with PBS, added with trypsin-EDTA digestion solution, and digested at 37° C. for 5 minutes. Pipette the cells, move to a centrifuge tube, and centrifuge at 800-1000r / min for 5min. Remove the digestion solution, add pre-cooled PBS, pipette to disperse the cells into a single-cell suspension as much as possible, centrifuge, repeat washing with cold PBS and resuspending the cells. Fix with 3.7% paraformaldehyde at 4° C. for 30 min, wash with PBS, and stain the cells with 50 μg / ml DAPI (containing 100 μg / ml RNase A) in the dark for 30 min. Wash twice with PBS, smear, observe the cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com