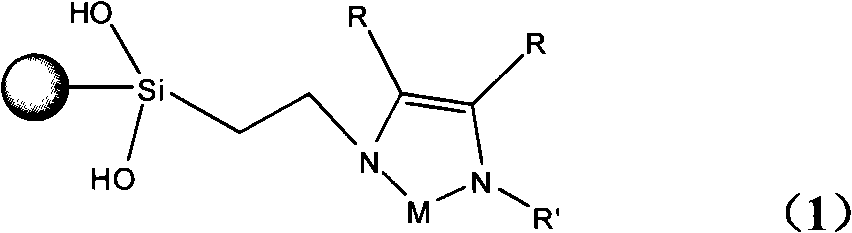
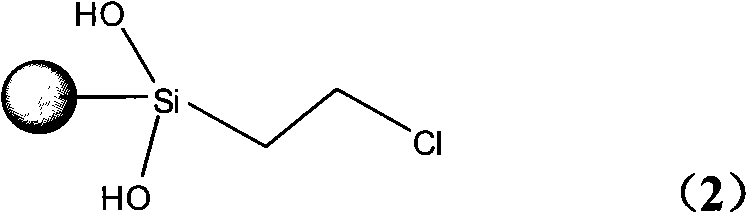
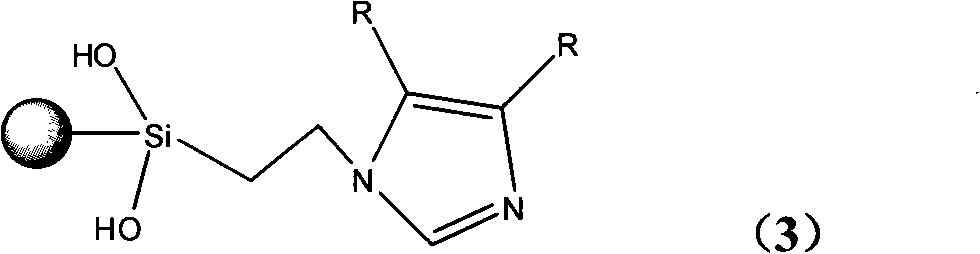
Glyoxaline ligand metallic catalyst supported on silica-gel and process for synthesizing the same
A metal catalyst and imidazole-loaded technology, which is applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve difficult industrial applications and conditions Harsh, complex operation and other problems, to achieve the effect of not easy to fall off, reduce usage, and reduce processing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] a. Silica gel activation: take 200 mesh column chromatography silica gel and put it in an oven, and bake it at 150°C for 10 hours to obtain activated silica gel. The elemental analysis results are shown in Table 1; the infrared analysis results are as follows:
[0028] IR(KBr) v: 3444.15, 1708.72, 1632.94, 1099.11, 970.78, 800.72, 469.21cm -1 .
[0029] b. Synthesis of silica gel-supported γ-chloropropyltrimethoxysilane (coupling agent): get 35g of activated silica gel and add it to 120ml of anhydrous xylene solution, then add 37ml of γ-chloropropyltrimethoxysilane, Heated and refluxed in an oil bath at 140°C for 72 hours. After the reaction, the mixture was filtered, washed with absolute ethanol, dried naturally, and vacuum-dried at 100°C to connect silica gel and imidazole together to obtain the structural formula ( 2) The light yellow-brown product ① shown is 42g. Yield 60.56%.
[0030] The elemental analysis results are shown in Table 1.
[0031] Table 1. Elemen...
Embodiment 2
[0048] Change the silica gel activation temperature in step a. in Example 1 to 160°C, and replace the imidazole in step c with 4,5-dimethyl-substituted imidazole. The yield of the product synthesized in this step is 92.5%, and the acetic acid added in step e 2g, other synthesis steps are the same as in Example 1. The palladium content of the final product was analyzed to be 3.50%.
Embodiment 3
[0050]Change the silica gel activation temperature of step a. in Example 1 to 170°C, change the palladium salt of step e into nickel salt nickel dichloride, the addition amount is 2g, potassium hydride 0.625g is used for alkaline substances, and cyclohexane is selected as the organic solvent alkane, refluxed at 80°C for 6 hours, and other synthesis steps were the same as in Example 1, and the nickel content of the final product was analyzed to be 3.60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com