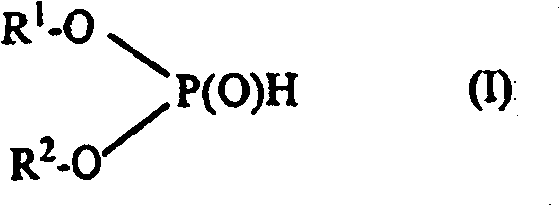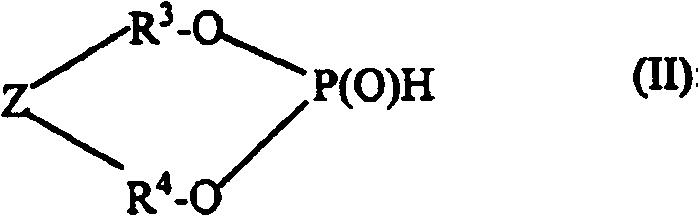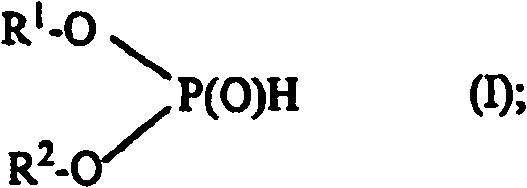Use of phosphonic acid diesters and diphosphonic acid diesters and thermally curable mixtures containing phosphonic acid diesters and diphosphonic acid diesters
A technology of diphosphonic acid diester and phosphonic acid diester, which is applied in the field of new curable mixtures, and can solve problems such as harmful storage stability and unfavorable viscosity of heat curable mixtures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Production of two-component systems 1 and varnishes 1
[0131] First, the two-component system 1 is produced by mixing the individual components of components (I) and (II) in the stated amounts and homogenizing the resulting mixture.
[0132] Component (I):
[0133] - 41.15 parts by weight of hydroxyl-containing polyester (from Bayer AG's Desmophen A870, 70% in butyl acetate),
[0134] -11.21 parts by weight amino resin (Cymel from Cytec Company 202),
[0135] -5.33 parts by weight of the first light stabilizer (Tinuvin available from Ciba Specialty Chemicals 292, 10% in butyl acetate);
[0136] - 10.67 parts by weight of the second light stabilizer (Tinuvin available from Ciba Specialty Chemicals 1130, 10% in butyl acetate);
[0137] -10.02 parts by weight methoxypropyl acetate (MPA) / Solvesso 100 (1:1) and -2.13 parts by weight of butyl glycol acetate.
[0138] Component (II):
[0139] - 19.49 parts by weight polyisocyanate (Basonat available from BA...
Embodiment 2
[0143] Production varnish coating 1
[0144] Use of steel plates coated with cathodically deposited and thermally cured electrocoats and thermally cured surfacers (Bonder 26S60) Production of varnish coating 1.
[0145] The commercially available aqueous basecoat material Brillantschwarz from the company BASF Coatings AG was applied over the surfacer. The resulting aqueous basecoat film was dried at 80°C for 10 minutes. Clearcoat material 1 of Example 1 was then applied, after which the aqueous basecoat film and clearcoat film 1 were co-cured at 140° C. within 22 minutes. This gave a basecoat with a film thickness of 10 μm and a clearcoat 1 with a film thickness of 40 μm.
[0146] Clearcoat 1 is completely transparent, free from paint defects such as blisters, spots or pits, bright and has a high gloss, no yellowing, hard, flexible, scratch-resistant, chemical-resistant, etch-resistant and resistant grind. It is therefore eminently suitable as a clearcoat within the rang...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com