Method for preparing cefozopran hydrochloride, cefozopran hydrochloride powder injection and preparation method thereof
A technology for cefazolam hydrochloride and injection, which is applied in the field of drug synthesis and its preparation, can solve the problems of complicated process, difficult preparation dissolution, poor stability and the like, and achieves the effects of high reaction yield, good fluidity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] [Example 1] Preparation of Cefozopran Hydrochloride
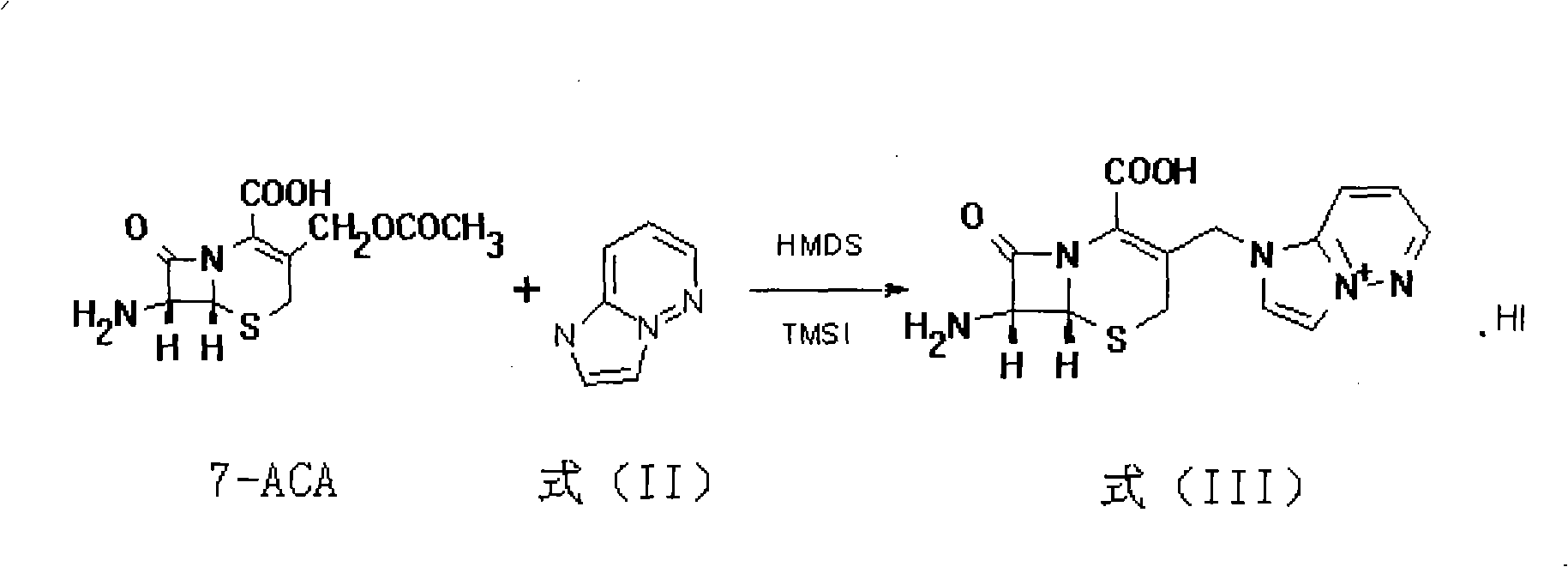
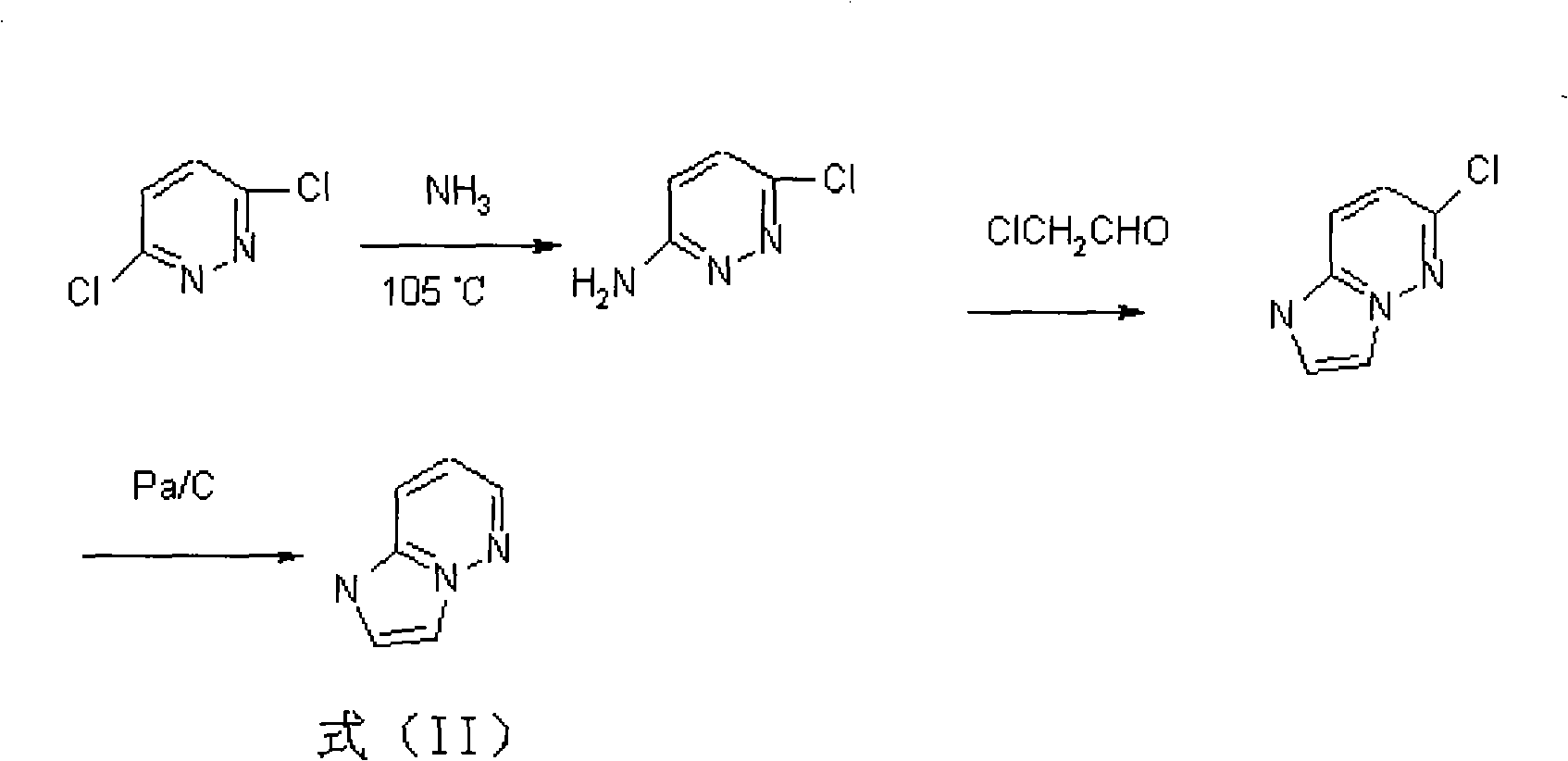
[0051] (1) Put 1.93Kg (7.6mol) of 7-ACA, 180ml of HMDS, 12L of dichloromethane and 0.1L of iodotrimethylsilane into a 100L reactor, heat and reflux for 8 hours, cool down to 0°C, and add iodotrimethylsilane 1.16L (14.5mol) was stirred for 8 hours, 2.9kg (27.1mol) of the compound of formula (II) was added with 1H-imidazol[1,2-b]pyridazine, stirred at room temperature for 8 hours, methanol was added dropwise, and the filter cake was dichloromethane Washing, washing with methanol, and drying in vacuo to give 7-amino--3-(1H-imidazo[1,2-b]pyridazin-4-ium-1-yl)methyl-8-oxo-5-thio- 1-azabicyclo[4.2.0]octyl-2-enyl-2-carboxylic acid.hydriodide is formula (III) compound ( figure 1 ) 3.1kg (yield = 85.6%), TLC inspection impurity spot is no more than 5% (developing agent: acetonitrile: water = 4: 1);
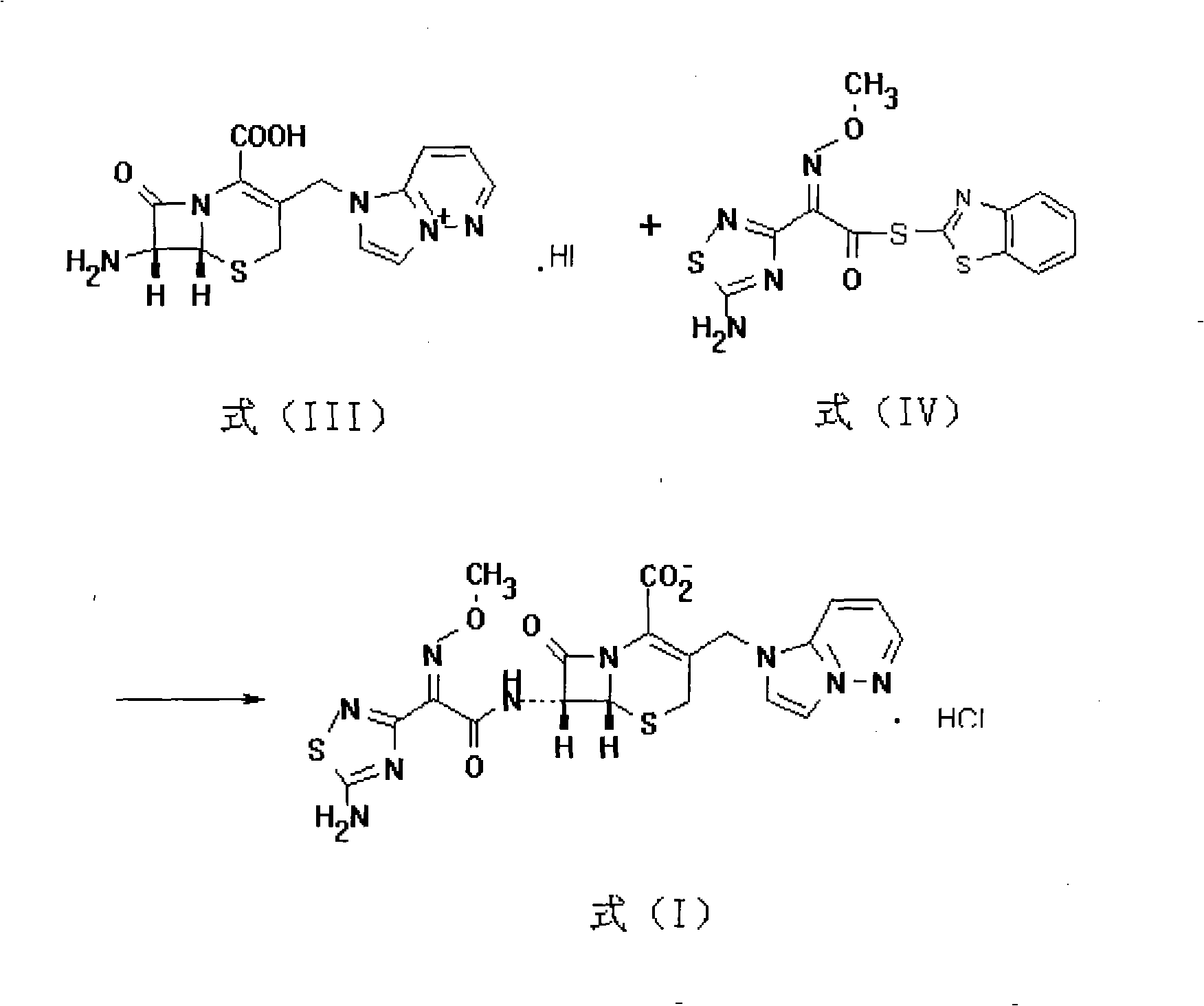
[0052] (2) Take the 7-amino-3-(1H-imidazo[1,2-b]pyridazin-4-ium-1-yl)methyl-8-oxo-5-thio- 1-Azabicyclo[4.2.0]octyl-2-enyl-2-...
Embodiment 2
[0054] [Embodiment 2] Preparation of Cefozopran Hydrochloride
[0055] (1) Put 1.93Kg (7.6mol) of 7-ACA, 180ml of HMDS, 12L of dichloromethane and 0.1L of iodotrimethylsilane into a 100L reactor, heat and reflux for 6 hours, cool down to 5°C, and add iodotrimethylsilane 1.16 L (14.5 mol) was stirred for 6 hours, 2.9 kg (27.1 mol) of the compound of formula (II) was added with 1H-imidazol[1,2-b] pyridazine, stirred at room temperature for 6 hours, methanol was added dropwise, and the filter cake was dichloromethane Washing, washing with methanol, and drying in vacuo to give 7-amino--3-(1H-imidazo[1,2-b]pyridazin-4-ium-1-yl)methyl-8-oxo-5-thio- 1-azabicyclo[4.2.0]octyl-2-enyl-2-carboxylic acid.hydriodide is formula (III) compound ( figure 1 ) 3.1kg (yield = 85.6%), TLC inspection impurity spot is no more than 5% (developing agent: acetonitrile: water = 4: 1);
[0056] (2) Take the 7-amino-3-(1H-imidazo[1,2-b]pyridazin-4-ium-1-yl)methyl-8-oxo-5-thio- 1-Azabicyclo[4.2.0]octyl-2...
Embodiment 3
[0058] [Example 3] Preparation of Cefozopran Hydrochloride
[0059](1) Put 1.93Kg (7.6mol) of 7-ACA, 180ml of HMDS, 12L of dichloromethane and 0.1L of iodotrimethylsilane into a 100L reactor, heat and reflux for 10 hours, cool down to 8°C, and add iodotrimethylsilane 1.16L (14.5mol) was stirred for 10 hours, 2.9kg (27.1mol) of the compound of formula (II) was added with 1H-imidazol[1,2-b]pyridazine, stirred at room temperature for 10 hours, methanol was added dropwise, and the filter cake was dichloromethane Washing, washing with methanol, and drying in vacuo to give 7-amino--3-(1H-imidazo[1,2-b]pyridazin-4-ium-1-yl)methyl-8-oxo-5-thio- 1-azabicyclo[4.2.0]octyl-2-enyl-2-carboxylic acid.hydriodide is formula (III) compound ( figure 1 ) 3.1kg (yield = 85.6%), TLC inspection impurity spot is no more than 5% (developing agent: acetonitrile: water = 4: 1);
[0060] (2) Take the 7-amino-3-(1H-imidazo[1,2-b]pyridazin-4-ium-1-yl)methyl-8-oxo-5-thio- 1-Azabicyclo[4.2.0]octyl-2-enyl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com