Method for extracting naringenin from Chinese medicament chinaroot greenbrier and application thereof
A technology of naringenin and sarsaparilla, which is applied in the field of extracting naringenin, can solve the problems of decreased nerve transmission speed, increased osmotic pressure, and complications of diabetes, and achieves remarkable effects, clear structure, and favorable quality control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Separation, extraction and purification of monomers
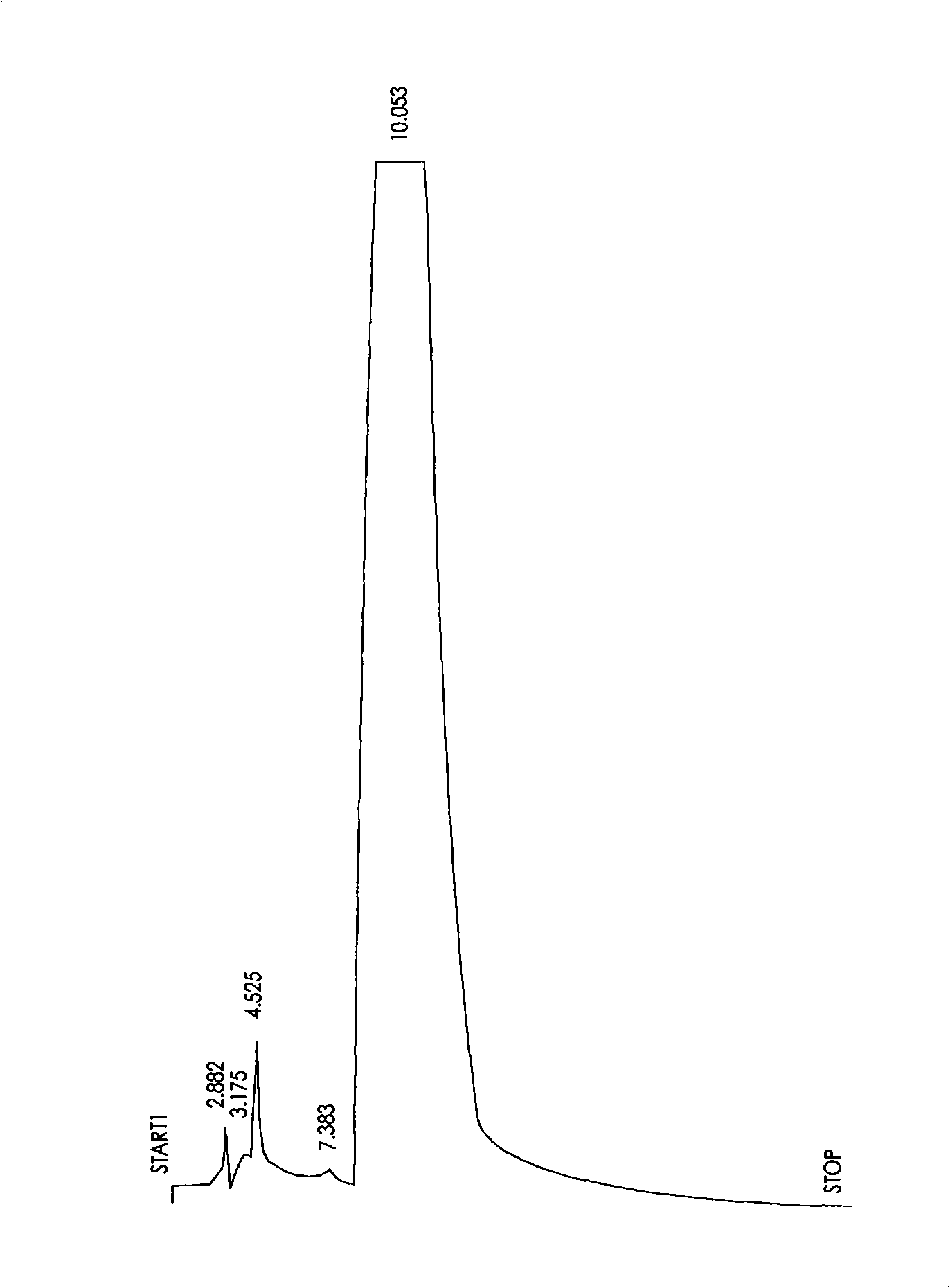
[0018] Weigh 1000g of Smilax sarsaparillae and extract it with 70% ethanol to obtain 78.32g of extract, which is then extracted successively with petroleum ether and ethyl acetate, and the ethyl acetate phase is 31.26g. Phase chromatography, mobile phase is ethyl acetate-methanol (9: 1), fractional collection, 200mL / bottle, 28 bottles are collected in total, and No. 4-12 bottles are combined and concentrated, and then put on a 200-300 mesh silica gel column ( 4.5×50cm), mobile phase chloroform-methanol (100:1) to chloroform-methanol (4:1) gradient elution, fractional collection, 200mL / bottle, 16 bottles were collected, 12, 13 bottles were concentrated, precipitated out . The precipitate can be subjected to single-gradient column chromatography depending on the purity, and the reference elution system is chloroform-methanol (9:1). Depending on the results of thin layer chromatography combined with ultravi...
Embodiment 2
[0021] Example 2: Structural Identification of Monomers
[0022] White needle-like crystals (methanol). Soluble in methanol, ethanol. Molecular weight is 272, molecular formula C 15 h 12 o 5 . . ESI-MSm / z: 273[M-H] + . 1 H NMR (CD 3 OD) δ: 7.36 (2H, d, J = 8.5, C 2 ’-H, C 6 '-H), 6.82 (2H, d, J=2.7, 9.4, C 3 ’-H, C 5 '-H), 5.98 (1H, d, J=2.1, C 6 -H), 5.35 (1H, dd, J=3.0, 13.5, C 2 -H), 3.05 (1H, d, J=14.5, C 3 -H α ), 2.78 (1H, dd, J=3.0, 14.6, C 3 -H β ). 13 C NMR (CD 3 OD) δ: 79.1 (C-2), 42.6 (C-3), 196.3 (C-4), 164.1 (C-5), 127.6 (C-6), 167.1 (C-7), 94.8 (C- 8), 163.5(C-9), 101.9(C-10), 129.7(C-2'), 115.0(C-3'), 157.6(C-4'), 115.0(C-5'), 127.6 (C-6').
[0023] In summary, the compound is 3(S)-5,7,4'-trihydroxydihydroflavone, namely naringenin (Naringenin, Nar).
Embodiment 3
[0024] Embodiment three: the inhibitory activity of monomer to α-glucosidase
[0025] Add 0.76mL of pH6.8 potassium phosphate buffer solution, 20μL of 1mg / mL reduced glutathione, 10μL of 0.0167U / mL α-glucosidase to the test tube in turn, after incubation at 37°C for 10min, add 10μL of 0.116M PNPG, Scan at a wavelength of 400nm for 6min to record the change of absorbance. The definition of α-glucosidase activity unit: under the conditions of pH 6.8 and temperature 37°C, at 400nm, the absorbance value rises by 0.1 per minute as an activity unit.
[0026] Add potassium phosphate buffer, 20 μL 1mg / mL reduced glutathione, 10 μL 0.0167U / mL α-glucosidase, and a certain amount of inhibitor solution (1mg / mL, prepared with potassium phosphate buffer), 37°C After incubation for 10 minutes, add 10 μL of 0.116M PNPG, scan at 400 nm wavelength for 6 minutes, and record the change of absorbance. The total reaction system is 0.8 mL. Definition of activity unit of α-glucosidase inhibitor: un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com