A method for making anode material LiFePO4 of lithium ion battery
A technology for lithium iron phosphate and lithium ion batteries, which is applied in the direction of electrode manufacturing, battery electrodes, chemical instruments and methods, etc., and can solve the limitations of large-scale industrialization of lithium iron phosphate, poor high-current discharge capacity of synthetic materials, and control conditions Harsh and other problems, to achieve the effect of reducing synthesis cost, good high current, and short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
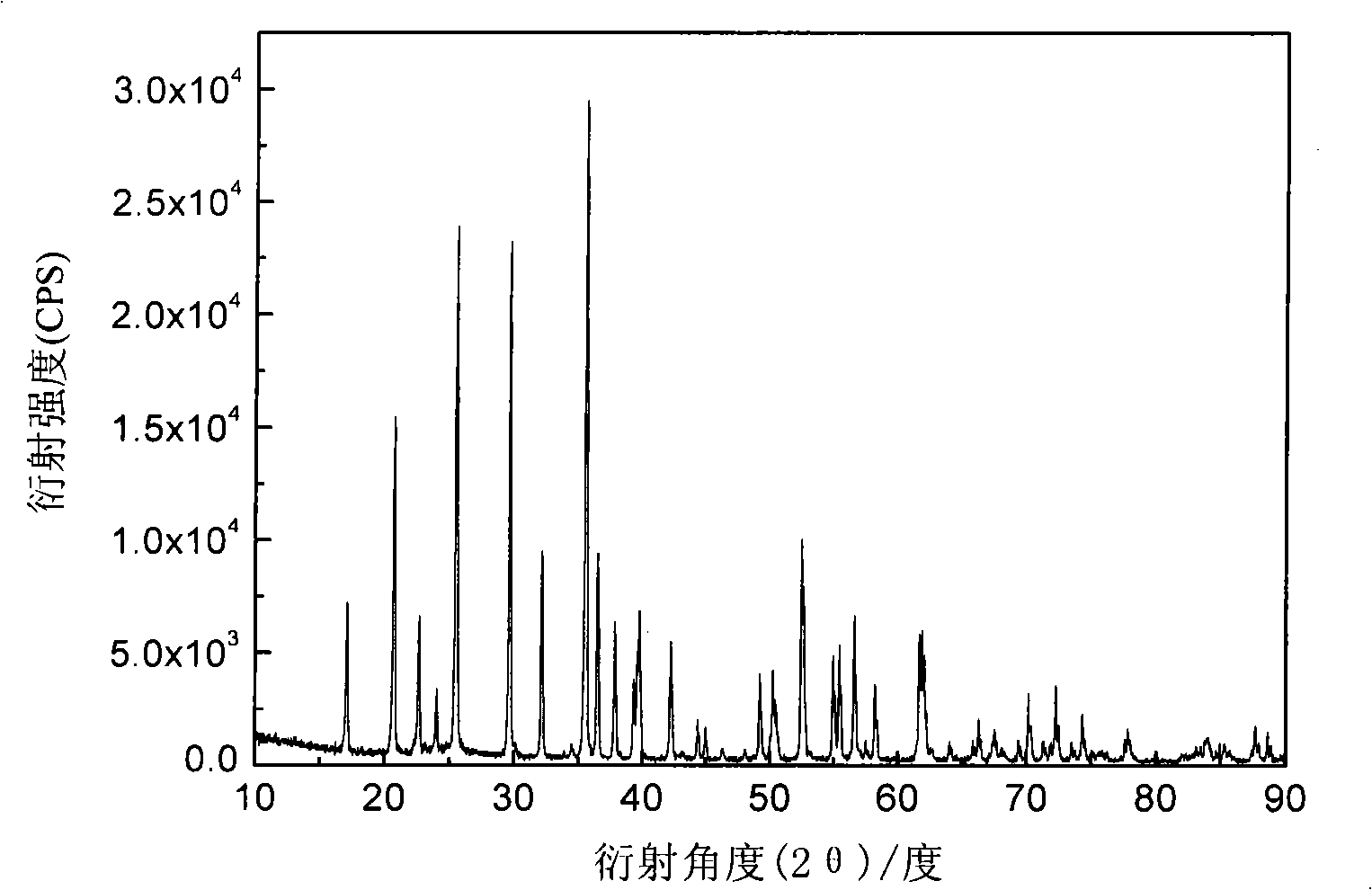
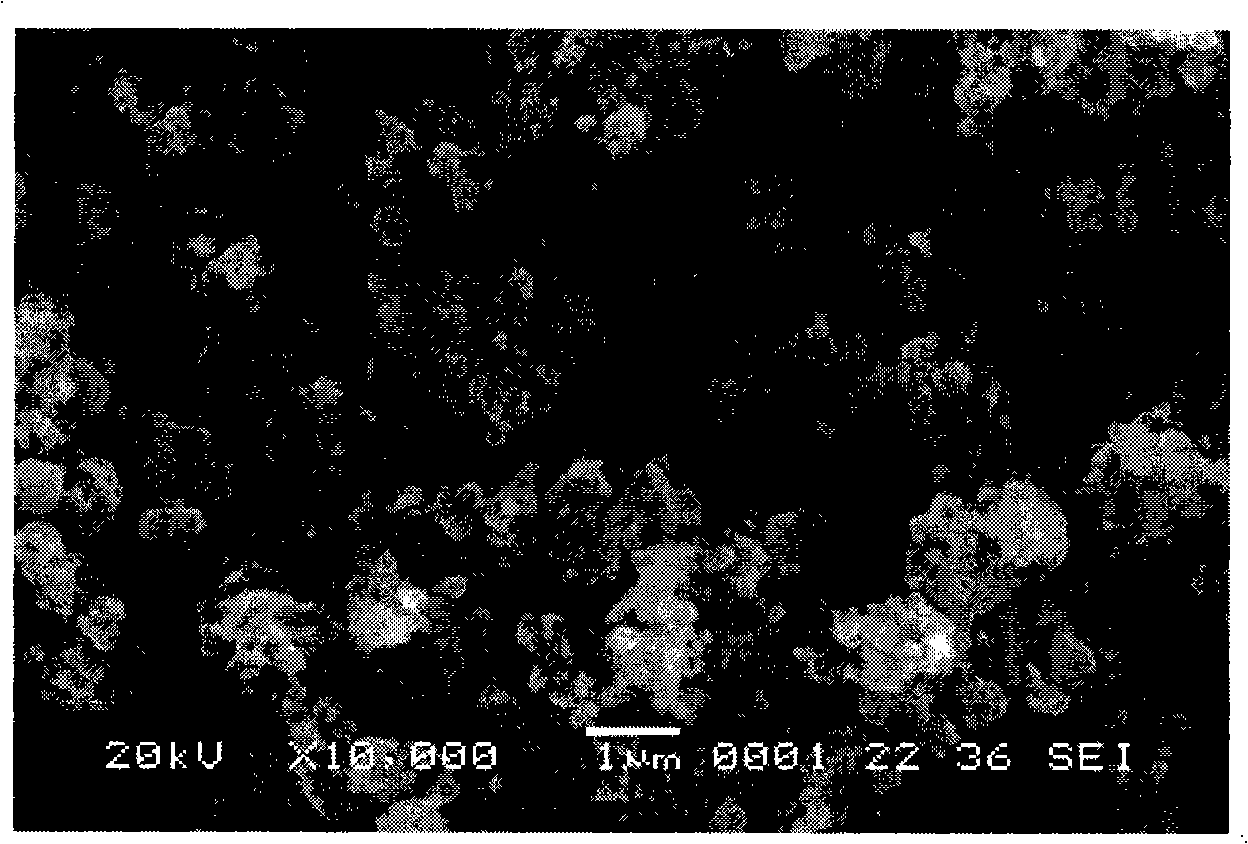
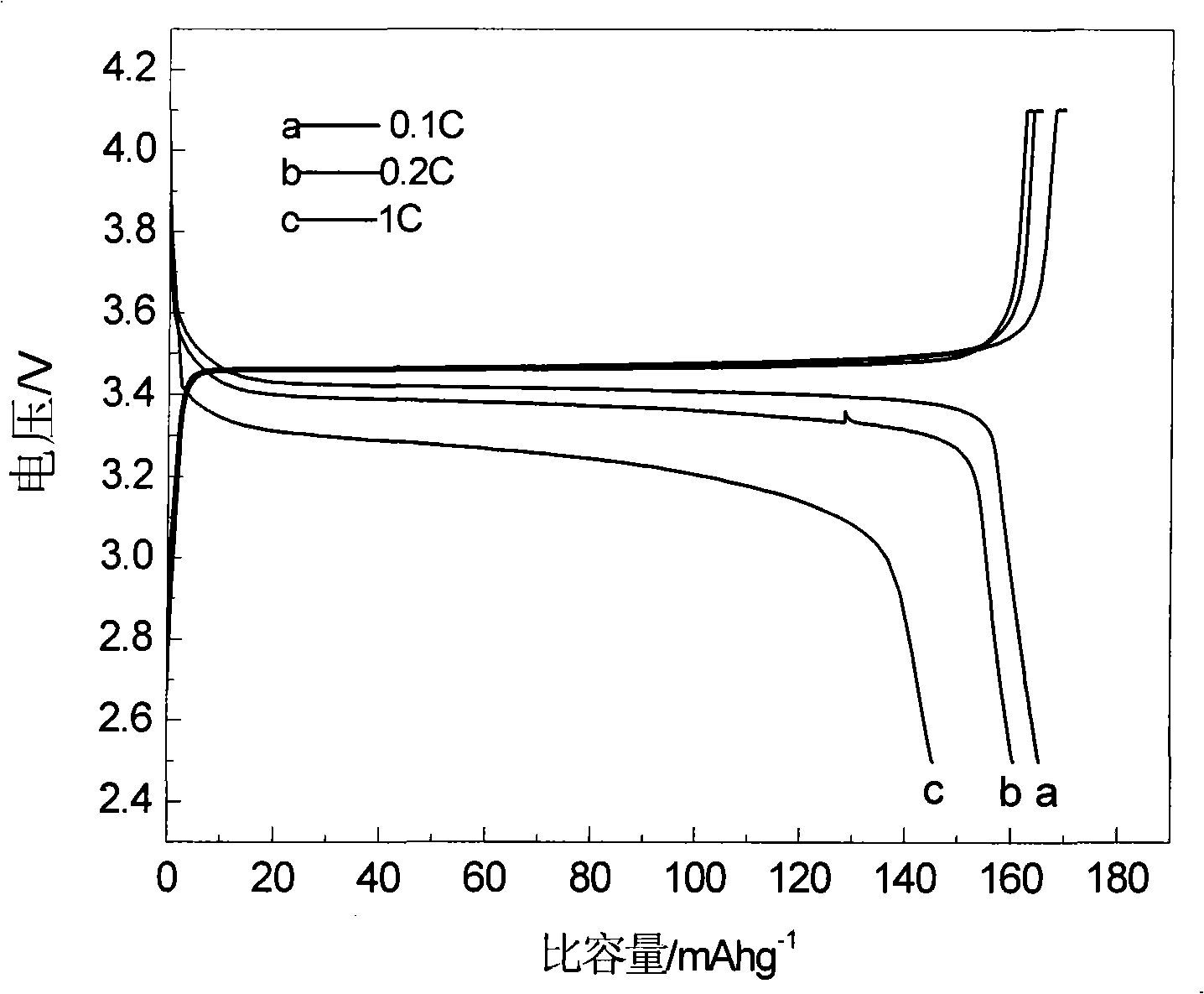
[0019] Ferric phosphate, lithium carbonate, malic acid are used as raw materials, mixed uniformly in a molar ratio of 1:0.5:1, and mechanically activated for 0.5 hours; then loaded into a tube furnace, under an argon atmosphere, the temperature is 300 ° C, ℃, 560℃, 700℃ for 12 hours. The obtained material is analyzed by X-ray diffraction as an olivine structure, and the space group is Pnma, which is LiFePO 4 Structure. The particle size of the product obtained by SEM is about 200nm. The obtained products were assembled into button batteries to measure their charge-discharge specific capacity and cycle performance. Charge and discharge were carried out at a rate of 0.1C. Their initial discharge capacity and discharge capacity after 50 cycles are shown in Table 1.
[0020] Experimental condition and result of table 1 embodiment 1
[0021]
Embodiment 2
[0023] Ferric nitrate, lithium formate, triammonium phosphate, and mandelic acid are used as raw materials, mixed uniformly in a molar ratio of 1:1:1:3, and mechanically activated for 20 hours; then put into a tube furnace, under a hydrogen atmosphere, the temperature Keep the temperature at 600°C for 2 hours, 5 hours, 8 hours, and 20 hours, respectively. The obtained material is analyzed by X-ray diffraction as an olivine structure, and the space group is Pnma, which is LiFePO 4 Structure. The particle size of the product obtained by SEM is about 200nm. The obtained products were assembled into button batteries to measure their charge-discharge specific capacity and cycle performance. Charge and discharge were carried out at a rate of 0.1C. Their initial discharge capacity and discharge capacity after 50 cycles are shown in Table 2.
[0024] Experimental condition and result of table 2 embodiment 2
[0025]
Embodiment 3
[0027] Using iron carbonate, lithium oxide, diammonium hydrogen phosphate, and oxalic acid as raw materials, mix them uniformly in a molar ratio of 1:1:2:4, and mechanically activate them for 8 hours; then put them into a tube furnace, and under a nitrogen atmosphere, The temperature was kept at 560°C for 15 hours. The obtained material is analyzed by X-ray diffraction as an olivine structure, and the space group is Pnma, which is LiFePO 4 Structure. The obtained product was assembled into a button battery to measure its charge-discharge specific capacity and cycle performance, and the charge-discharge was carried out at a rate of 0.1C, and the initial discharge capacity was 165mAh·g -1 , the discharge capacity after 50 cycles is 165mAh·g -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com