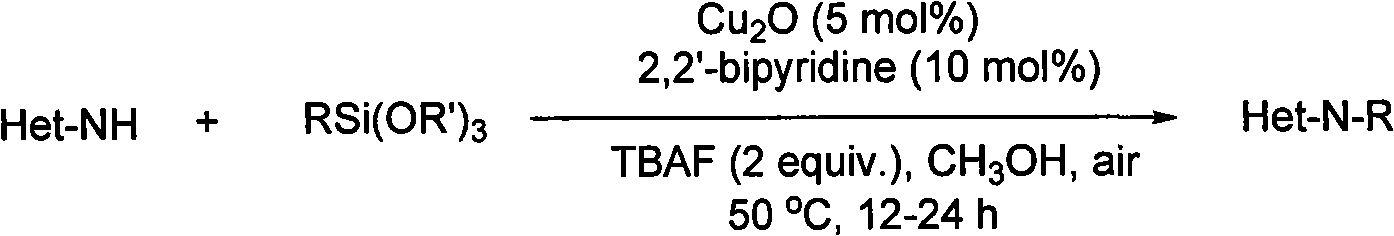Preparation method for N-arylazoles compounds
A technology of azole compounds and compounds, applied in the field of compound preparation, can solve the problems of only 69% yield, low yield, and narrow scope of substrate application, and achieve the effects of low price, simple operation, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Cu was added sequentially to a 25mL single-neck round bottom flask 2 O (4 mg, 5 mol%), 2,2'-bipyridine (8 mg, 10 mol%), methanol (3 mL), TBAF (1.0 mmol) in THF (1 M, 1 mL), phenyltrimethoxysilane (1 mmol) and Benzimidazole (0.5mmol), connected to a condensing reflux tube at the bottle mouth, placed the device in a 50°C oil bath, stirred magnetically, reacted for 12h, removed the solvent under reduced pressure, and carried out column separation with petroleum ether / ethyl acetate to obtain the product , NMR to determine the structure. Yield was 99%.
[0020] 1 H NMR (400MHz, CDCl 3 , TMS)δ8.04(s, 1H), 7.86-7.88(d, 1H, J=7.2Hz), 7.44-7.49(m, 3H), 7.35-7.39(m, 3H), 7.23-7.31(m, 2H). MS (EI): m / z (%): 194(100) [M + ], 193(24)[M + -1].
Embodiment 2
[0022] Cu was added sequentially to a 25mL single-neck round bottom flask 2 O (4 mg, 5 mol%), 2,2'-bipyridine (8 mg, 10 mol%), methanol (4 mL), TBAF (1.0 mmol) in THF (1 M, 1 mL), phenyltrimethoxysilane (1 mmol) and 5,6-Dimethylbenzimidazole (0.5mmol), connect the condensing reflux tube to the bottle mouth, place the device in a 50°C oil bath, stir magnetically, react for 24h, remove the solvent under reduced pressure, use petroleum ether / ethyl acetate The ester was subjected to column separation to obtain the product, and the structure was determined by NMR. Yield was 89%.
[0023] 1 H NMR (400MHz, CDCl 3 , TMS)δ8.00(s, 1H), 7.63(s, 1H), 7.53-7.57(t, 2H), 7.41-7.49(m, 3H), 7.31(s, 1H), 2.39(s, 3H) , 2.37 (s, 3H). MS (EI): m / z (%): 222 (100) [M + ], 221(51)[M + -1], 207(55), 77(7).
Embodiment 3
[0025] Cu was added sequentially to a 25mL single-neck round bottom flask 2 O (7 mg, 5 mol%), 2,2'-bipyridine (16 mg, 10 mol%), methanol (6 mL), TBAF (2.0 mmol) in THF (1 M, 2 mL), phenyltrimethoxysilane (2 mmol) and Imidazole (1.0 mmol), connected to a condensing reflux tube at the bottle mouth, placed the device in a 50°C oil bath, stirred magnetically, reacted for 12 h, removed the solvent under reduced pressure, and carried out column separation with petroleum ether / ethyl acetate to obtain the product. NMR Determine the structure. Yield was 98%.
[0026] 1 H NMR (400MHz, CDCl 3 , TMS)δ7.86(s, 1H), 7.46-7.49(t, 2H), 7.36-7.39(t, 3H), 7.27(s, 1H), 7.21(s, 1H). MS(EI): m / z(%): 144(100)[M + ], 117(43), 90(31), 77(23), 51(10).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com