Oxygen reduction electrocatalyst and preparation thereof
An electrocatalyst and carbon black technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of increasing fuel cell manufacturing costs, hindering commercial production, Complicated synthesis process and other issues, to achieve the effects of shortening the preparation time, strong controllability of the preparation process, and simplifying the reaction process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] At 0°C, add 40g of absolute ethanol and 0.0806g of cobalt chloride into a 100mL flask. After the cobalt chloride is completely dissolved, add 0.25g of triethylenetetramine dropwise under stirring, continue stirring for 10 minutes, and add 0.1g of carbon black carrier Vulcan XC-72R, continued to stir for 2 hours, evaporated to remove absolute ethanol, heat-treated at 800°C for 90 minutes under the protection of inert gas Ar, and then cooled to obtain an oxygen reduction electrocatalyst carbon-supported cobalt triethylenetetramine.
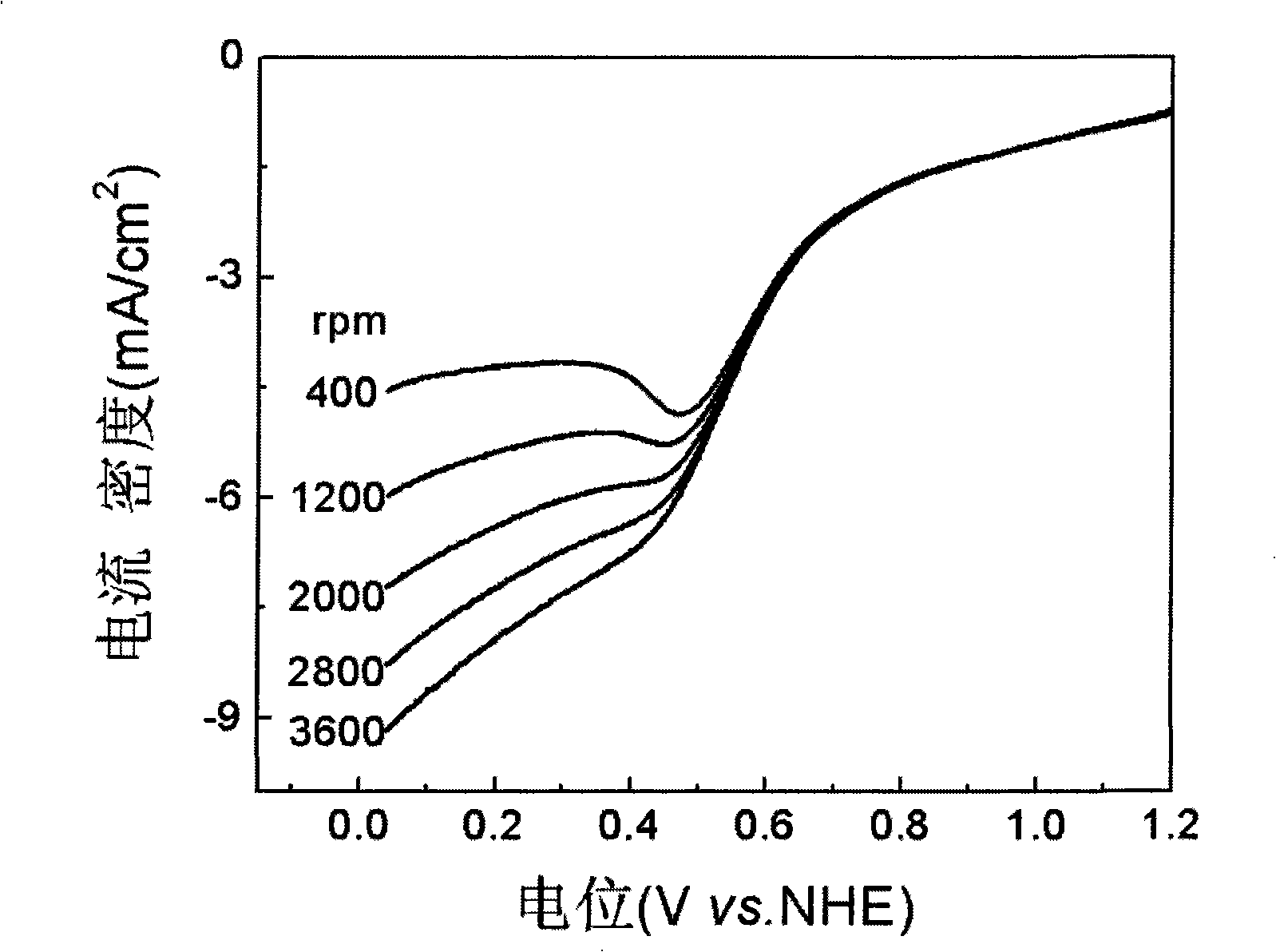
[0027] figure 1 It is the carbon-supported cobalt triethylenetetramine of the oxygen reduction electrocatalyst that embodiment 1 obtains in oxygen-saturated 0.5MH 2 SO 4 The cyclic voltammetry curve in the solution, the scanning speed is 50mV / s, there is a very obvious characteristic peak of oxygen reduction in the figure, the peak potential is 0.415V (vs.NHE), and the peak current is 13.05mA / cm 2 , indicating that the catalyst of the prese...
Embodiment 2
[0031]At 25°C, add 8g of absolute ethanol and 0.0403g of cobalt chloride into a 100mL flask. After the cobalt chloride is completely dissolved, add 0.1g of triethylenetetramine dropwise under stirring, continue stirring for 30 minutes, and add 0.1g of carbon black carrier Ketjen Black, continued to stir for 240 minutes, evaporated to remove absolute ethanol, protected by inert gas Ar and heat-treated at 800°C for 120 minutes at a high temperature, and cooled to obtain an oxygen reduction electrocatalyst with carbon-supported cobalt triethylenetetramine.
[0032] Figure 4 It is the carbon-supported cobalt triethylenetetramine of the oxygen reduction electrocatalyst that embodiment 2 obtains in oxygen-saturated 0.5MH 2 SO 4 The electrochemical cyclic voltammetry test was carried out in the solution, and the scanning speed was 50mV / s. It was found that the characteristic peak of oxygen reduction was obvious, and the peak potential was the highest at 0.6745V (vs. NHE), but the p...
Embodiment 3
[0034] At 50°C, add 80g of absolute ethanol and 0.0844g of cobalt acetate into a 100mL flask. After the cobalt acetate is completely dissolved, add 0.2 g of triethylenetetramine dropwise under stirring, continue stirring for 30 minutes, add 0.2 g of carbon black carrier Black Pearl 2000, and continue stirring for 240 minutes. Evaporation removes absolute ethanol, heats at 800°C for 60 minutes under the protection of inert gas Ar, and cools to obtain a carbon-supported cobalt triethylenetetramine as an oxygen reduction electrocatalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com