Quality control method of cow-bezoar antiphlogistic tablet
A quality control method and technology for anti-inflammatory tablets, applied in the field of quality control of Niuhuang anti-inflammatory tablets, can solve problems such as failure to supervise drug safety and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0021] Take 20 tablets of this product, remove the sugar coating, grind finely, add 40 ml of methanol, ultrasonically treat for 30 minutes, filter, and concentrate the filtrate to 5 ml as the test solution. Take another bile acid reference substance, add ethanol to make a solution containing 1 mg per 1 ml, as the reference substance solution. According to the test of thin-layer chromatography (Appendix VI B of the Chinese Pharmacopoeia in 2005), draw 10 microliters of each of the above two solutions, and place them on the same silica gel G thin-layer plate respectively, and use n-hexane-ethyl acetate-acetic acid-methanol -The upper layer solution of formic acid (20:25:2:3:0.1) is used as a developing agent, developed, taken out, dried, sprayed with 10% sulfuric acid ethanol solution, and heated at 105°C until the spots are clearly colored. In the chromatogram of the test product, there are spots of the same color at the position corresponding to the chromatogram of the referen...
experiment example 2
[0025] Take 15 tablets of this product, remove the sugar coating, grind finely, add 50 ml of chloroform, heat and reflux for extraction for 30 minutes, filter, and concentrate the filtrate to 1 ml as the test solution. Take another reference substance of indigo and indirubin, and add chloroform to make a mixed solution containing 1 mg per 1 ml, as the reference solution. According to the thin-layer chromatography (Appendix VI B of the Chinese Pharmacopoeia 2005 edition), draw 10 microliters of each of the above-mentioned three solutions, respectively spot on the same silica gel G thin-layer plate, and use chloroform-acetone (19:2) As a developing agent, unfold, take out, and let it dry. In the chromatogram of the test product, there are spots of the same color at the position corresponding to the chromatogram of the reference product.
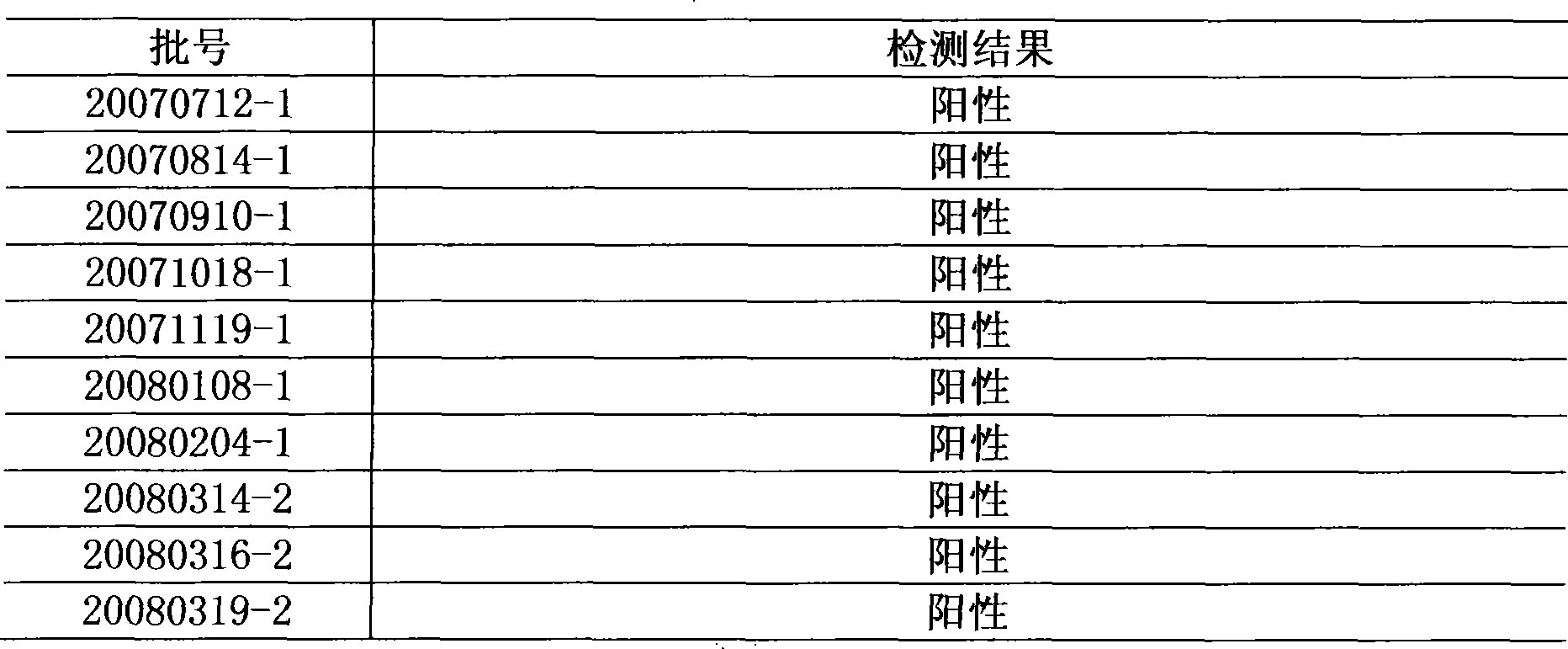
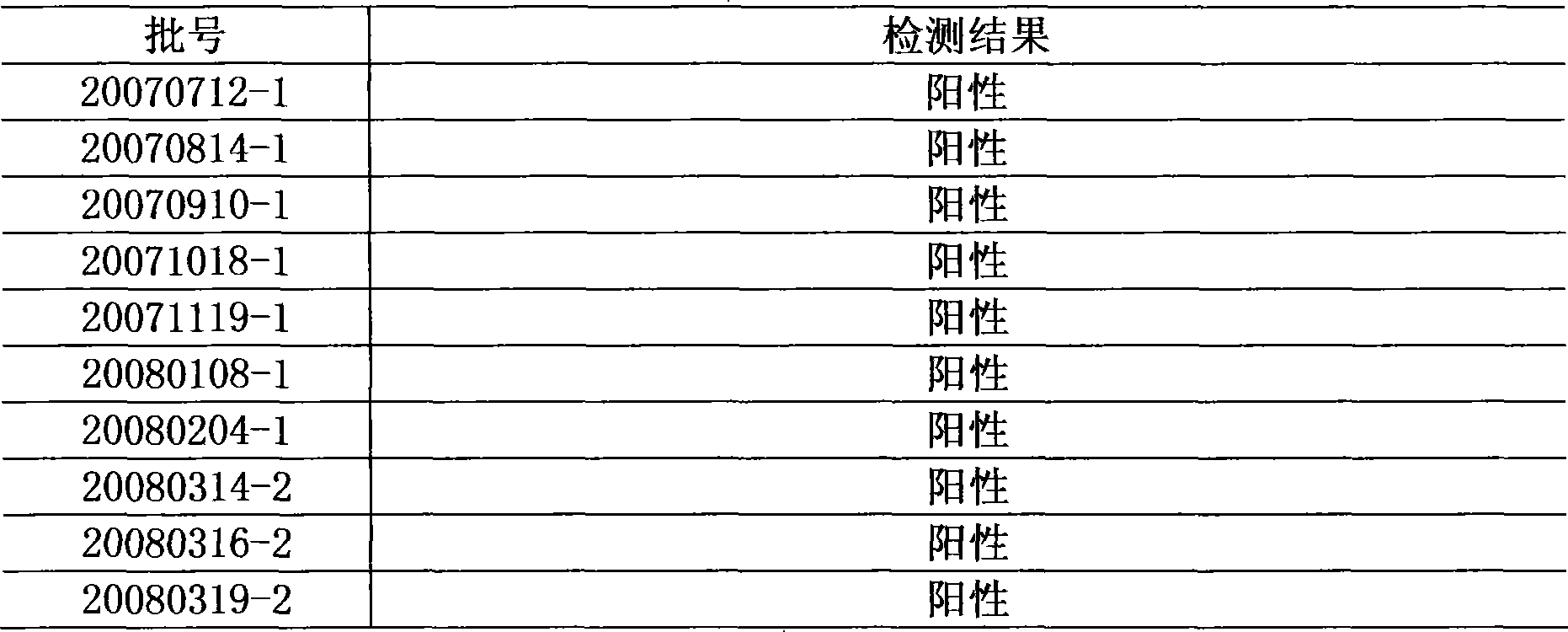
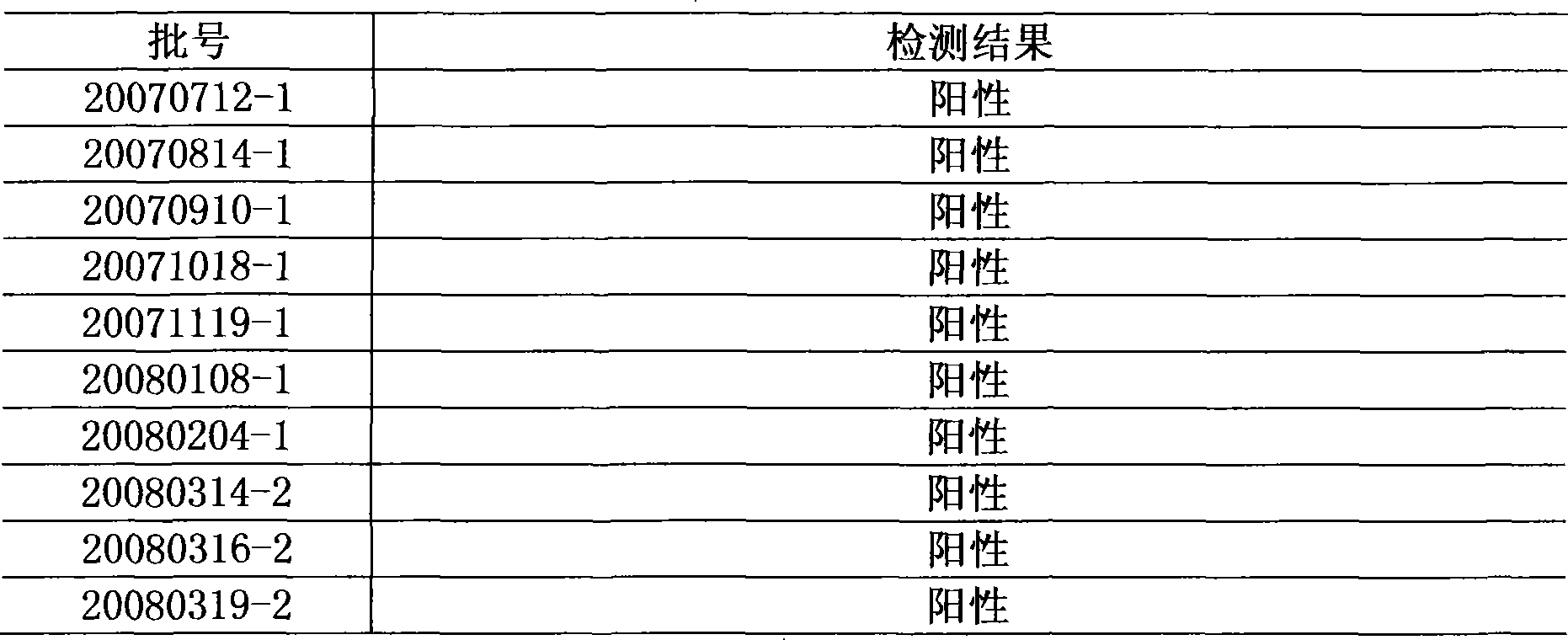
[0026] Using this method to repeat the test of 10 batches of Niuhuang Xiaoyan Tablets, all were positive.
[0027]
experiment example 3
[0029] Take 20 tablets of this product, remove the sugar coating, grind finely, add 30 ml of methanol, heat and reflux for extraction for 30 minutes, let cool, filter, evaporate the filtrate to dryness, add 15 ml of water to dissolve the residue, add 1 ml of hydrochloric acid, and place in a hot water bath Heat for 30 minutes, cool immediately, extract twice with ether, 10 ml each time, combine the ether extracts, evaporate to dryness, add 1 ml of ethyl acetate to the residue to dissolve, and use it as the test solution. In addition, take chrysophanol and emodin reference substances, add methanol to make a mixed solution containing 1 mg in each 1 ml, as the reference solution. According to the thin-layer chromatography (Appendix VI B of the Chinese Pharmacopoeia in 2005 edition), each 5 microliters of the above-mentioned two solutions were drawn, and they were respectively spotted on the same silica gel G thin-layer plate with carboxymethylcellulose sodium as the binding agent....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com