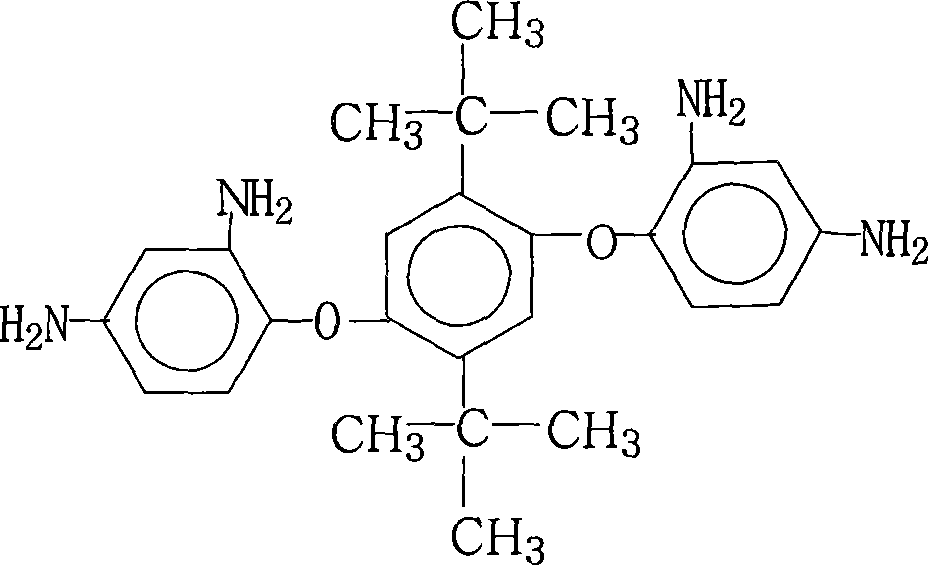
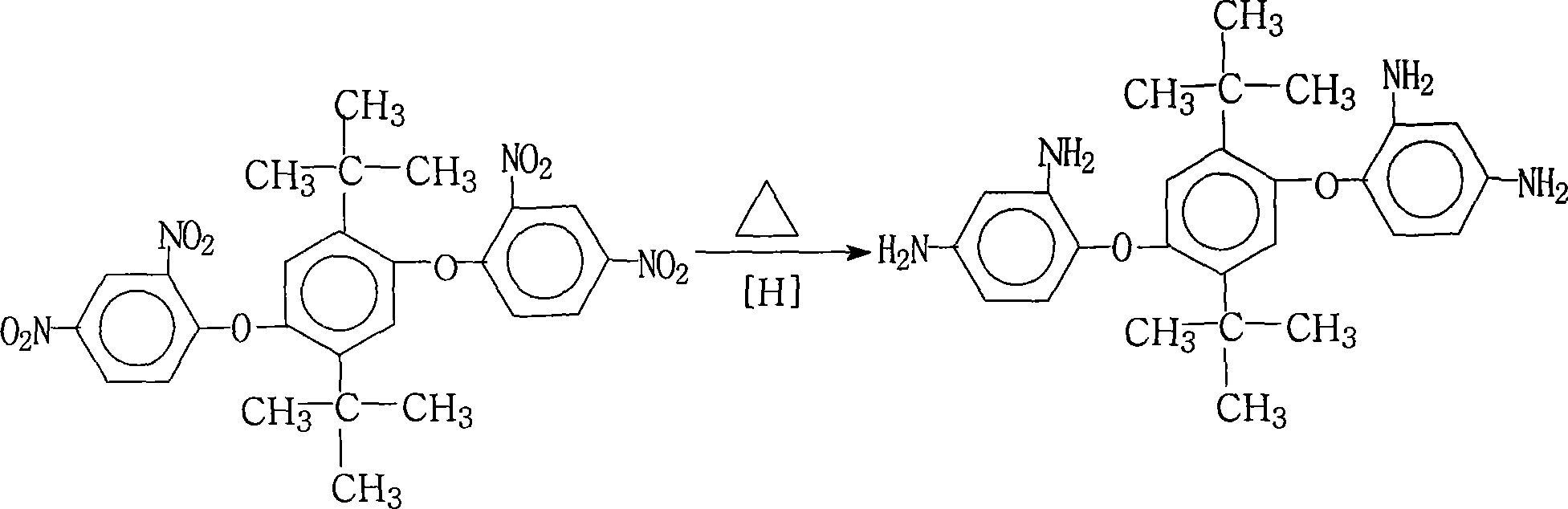
Process for producing 1,4-(2,4-diaminophenyloxy)-2,5-di-t-butyl benzene
A technology of diaminophenoxy and dinitrophenoxy, applied in 1 field, can solve the problems such as no patents, literature reports and the like in the preparation method, and achieve the effects of convenient source of raw materials, high yield and few chemical raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 55.4 grams (0.1 moles) of 1,4-bis(2,4-dinitrophenoxy)-2,5-di-tert-butylbenzene, 0.6 grams of palladium / carbon with a mass percentage of palladium of 15%, and 200 ml Ethanol and 670ml ethylene glycol were added in the reaction flask, stirred, heated up to 60° C., and began to add dropwise a mass concentration of 60% hydrazine hydrate solution (hydrazine hydrate molecular weight was 50.06), a total of 250.0 grams (solution weight). After the addition of hydrazine hydrate was completed, the stirring reaction was continued for 1 hour in the temperature range of 70°C to 85°C, cooled slightly, filtered while hot, cooling the mother liquor, adding pure water, and the crystalline product was precipitated, filtered, and vacuum-dried to obtain 41.0 g of 1 , 4-bis(2,4-diaminophenoxy)-2,5-di-tert-butylbenzene crystal, the purity is 99.5%, according to the actual obtained 1,4-bis(2,4-diaminophenoxy base)-2,5-di-tert-butylbenzene amount and theoretical amount (43.4 grams), calculate ...
Embodiment 2
[0029] 55.4 grams (0.1 moles) of 1,4-bis(2,4-dinitrophenoxy)-2,5-di-tert-butylbenzene, 11.0 grams of palladium mass percent is 1% palladium / carbon and 5540ml ethanol Add it into the reaction flask, stir, heat up to 60° C., and start to add dropwise a hydrazine hydrate solution with a mass concentration of 85%, totaling 294.1 grams (solution weight). After the dropwise addition of hydrazine hydrate was completed, the stirring reaction was continued for 5 hours at a temperature range of 70°C to 85°C, cooled slightly, filtered while hot, cooling the mother liquor, adding pure water, the crystallized product was precipitated, filtered, and vacuum-dried to obtain 40.6 grams of 1 , 4-bis(2,4-diaminophenoxy)-2,5-di-tert-butylbenzene crystal, the purity is 99.3%, according to the actual obtained 1,4-bis(2,4-diaminophenoxy base)-2,5-di-tert-butylbenzene amount and theoretical amount (43.4 grams), calculate 1,4-bis(2,4-diaminophenoxy)-2,5-di-tert-butylbenzene The yield was 93.5%.
Embodiment 3
[0031] 55.4 grams (0.1 mole) of 1,4-bis(2,4-dinitrophenoxy)-2,5-di-tert-butylbenzene, 4.0 grams of palladium mass percentage is 5% palladium / carbon and 1000ml 2 - Add methoxyethanol in the reaction flask, stir, heat up to 60° C., and start to add dropwise a hydrazine hydrate solution with a mass concentration of 80%, totaling 260.0 grams (solution weight). After the addition of hydrazine hydrate was completed, the stirring reaction was continued for 4 hours at a temperature range of 70°C to 85°C, cooled slightly, filtered while hot, cooling the mother liquor, adding pure water, and crystallized product was precipitated, filtered, and vacuum-dried to obtain 39.7 grams of 1 , 4-bis(2,4-diaminophenoxy)-2,5-di-tert-butylbenzene crystal, the purity is 99.4%, according to the actual obtained 1,4-bis(2,4-diaminophenoxy base)-2,5-di-tert-butylbenzene amount and theoretical amount (43.4 grams), calculate 1,4-bis(2,4-diaminophenoxy)-2,5-di-tert-butylbenzene The yield was 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com