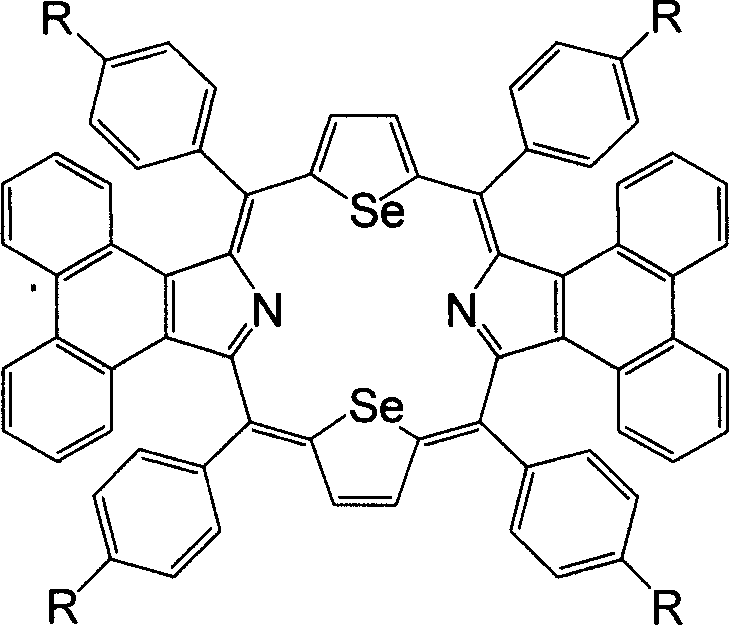
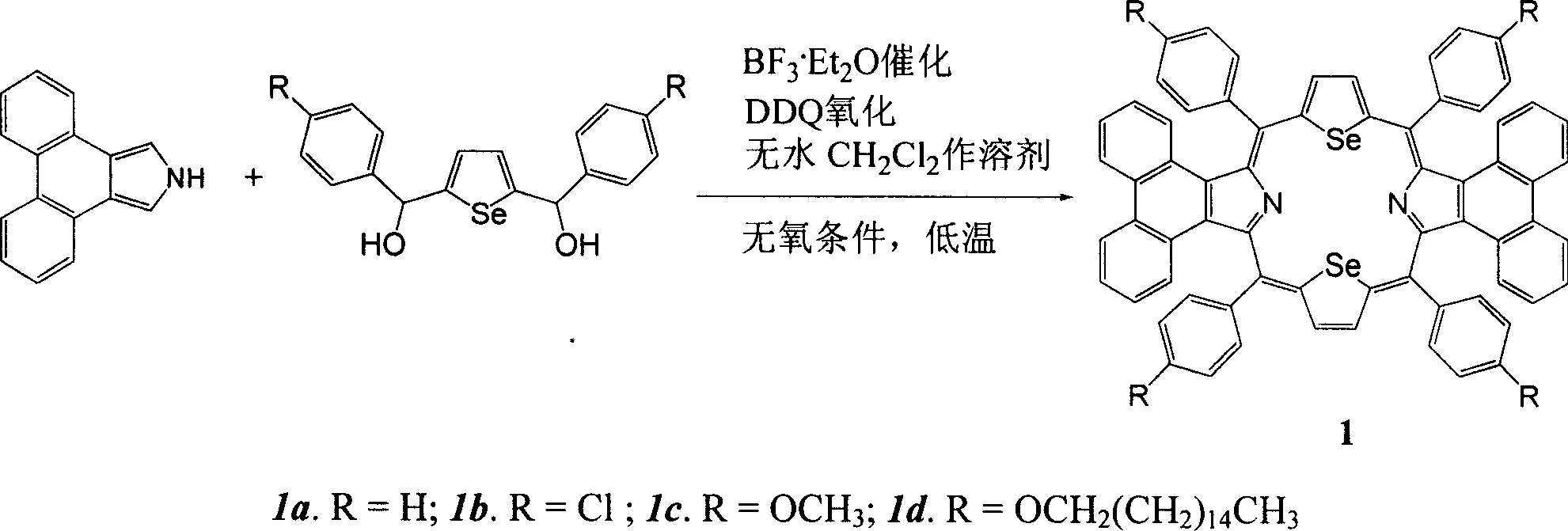
Synthesis of middle position-tetraaryldiphenanthrene diselenoporphyrin derivative and application thereof
A technology of tetraaryldiphenanthrene and tetraphenyldiphenanthrene, which is applied to the synthesis of meso-tetraaryldiphenanthrene and diselenite porphyrin derivatives and its application field, and can solve the problem of many by-products and low yield , purification difficulties and other problems, to achieve the effect of improving absorption efficiency and improving conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
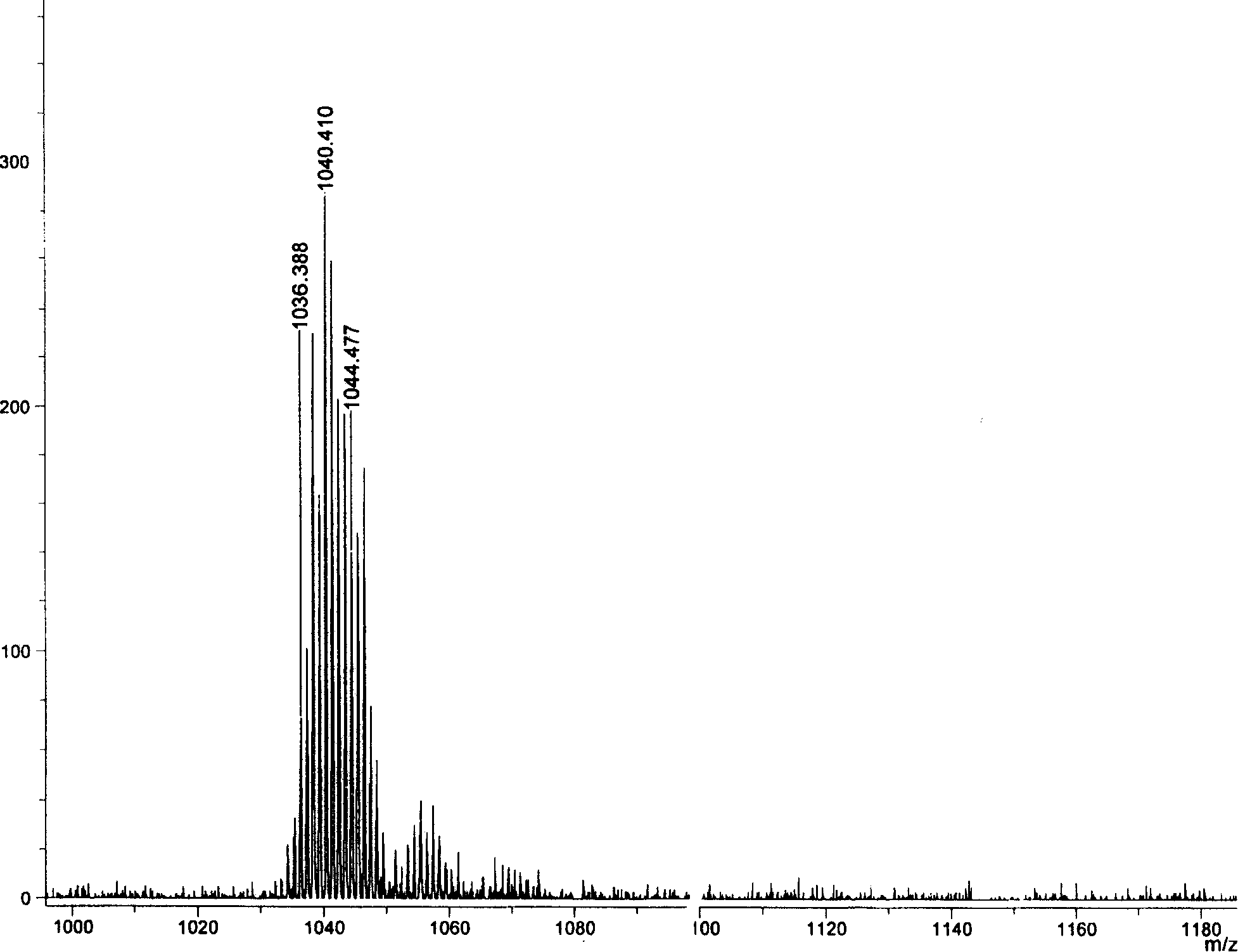
[0024] Example 1: 5,10,15,20-tetraphenyl-diphenanthrene[9,10-b:9,10-l]-22,24-diselenoporphyrin (1a) was synthesized in a 250ml circle Add 1mmol (278mg) 2,5-bis(phenylhydroxymethyl)selenophene, 1mmol (217mg) phenanthropyrrole and 60ml anhydrous dichloromethane in the bottom flask, put into the magnetron and start stirring, under the protection of argon, the Put the reaction bottle in a low temperature device and keep it away from light, control the reaction temperature at -50±5°C, add a total of 80 μl of BF 3 ·Et 2 O, after making it react at low temperature for 2 hours, allow it to naturally warm up to room temperature and continue the reaction for 48 hours. 1.0 mmol DDQ (227 mg) was added to the reaction solution, and reacted for 1 hour. The solvent was distilled off under reduced pressure for chromatographic separation, and dark green crystals were obtained after recrystallization from methanol and chloroform. Yield: 19.7%; Melting point: >250°C; MALDI-TOFMS: calcd for C ...
Embodiment 2
[0025] Example 2: 5,10,15,20-tetrakis(4-chlorophenyl)-diphenanthrene[9,10-b:9,10-l]-22,24-diselenoporphyrin (1b) The synthetic preparation method is the same as in Example 1, and the yield: 29.4%. Melting point: >250℃; MALDI-TOFMS: calcd for C 68 h 36 Cl 4 N 2 Se 2 1180.76, found: 1182.45 (M + )( Figure 5 ); 1 H-NMR (500MHz, CDCl 3 )δ8.67-8.69 (4H, m), 8.64 (4H, m), 8.40-8.42 (8H, m), 8.20-8.21 (4H, m), 7.69-7.70 (8H, m), 7.50-7.53 ( 4H, m), 7.17-7.20 (4H, m); UV-vis: 524nm, 598nm, 820nm ( Figure 6 ).
Embodiment 3
[0026] Example 3: 5,10,15,20-tetrakis(4-methoxyphenyl)-diphenanthrene[9,10-b:9,10-l]-22,24-diselenoporphyrin (1c ) The synthetic preparation method is the same as Example 1, productive rate: 43.9%. Melting point: >250℃; MALDI-TOFMS: calcd for C 72 h 48 N 2 o 4 Se 2 1163.08, found: 1162.80 (M + )( Figure 7 ); 1 H-NMR (500MHz, CDCl 3 )δ8.62-8.65 (8H, m), 8.42-8.43 (8H, m), 8.27-8.29 (4H, m), 7.42-7.45 (4H, m), 7.22-7.26 (8H, m), 7.12- 7.15(4H, m), 3.99(12H, s); UV-vis: 526nm, 605nm, 844nm ( Figure 8 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com