Total synthesis method of natural product barrenwort glycosides compounds
A technology of icariin and compound, applied in the field of medicine, can solve the problems of harsh reaction conditions, low reaction yield, insufficient research and the like, and achieves the effects of easy availability of raw materials, high yield and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
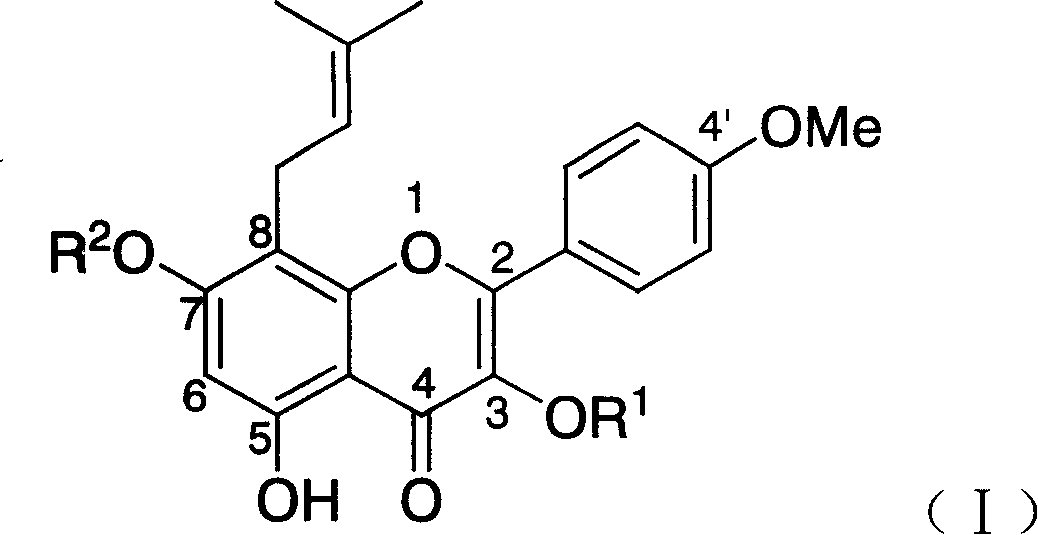
[0077] Example 1 synthetic compound A: dehydrated icariin (icariin, anhydroicartin), R 1 = H, R 2 =H
[0078] 1) Synthesis of chloromethyl anisole ii: mix benzyl alcohol (108g, 1.0mol) with paraformaldehyde (30g, 1.0mol by HCHO), keep the mixture at 0°C in an ice bath, quickly feed hydrochloric acid gas and Keep at 0°C with vigorous stirring for 4 hours. After stopping the stirring and warming to room temperature, the mixture was separated into layers, the upper organic phase was separated, 500ml of petroleum ether was added, dried over anhydrous magnesium sulfate for 1 hour and filtered off, a small amount of anhydrous calcium chloride was added and concentrated to obtain an oily substance. Distillation under reduced pressure gave 106 g of compound ii as a colorless liquid (70-72° C., 3 mmHg), with a yield of 68%.
[0079] 2) Synthesis of cyanomethyl anisole iii: Add cuprous cyanide (66 g, 0.74 mol) to the solution obtained by dissolving compound ii (106 g, 0.68 mol) in 12...
Embodiment 2
[0091] Example 2 Synthesis of Compound B: Icariside I (Icariside I), R 1 = H, R 2 =Glc
[0092] Synthesis of dehydroicariin-7-O-β-tetraacetyl-D-glucopyranoside xiii: Compound A dehydroicariin (1 g, 2.7 mmol) was added to dry pyridine (20 ml), stirred and dissolved Afterwards, silver nitrate (0.9g, 5.4mmol) and powdered 4A molecular sieves (2.0g) were added, and after stirring at room temperature in the dark for 1 hour, α-bromotetraacetyl-D-glucopyranose (1.22g, 2.9 mmol), after continuing to stir at room temperature in the dark for 48 hours, the reaction solution was diluted with 100ml chloroform, filtered through diatomaceous earth to remove silver salts and molecular sieves, and the filtrate was washed successively with 100ml1 mol / liter hydrochloric acid and 100ml saturated aqueous sodium chloride solution, anhydrous sulfuric acid After magnesium drying, filtration, and concentration, the residue was purified by silica gel column chromatography (gradient elution, eluent ra...
Embodiment 3
[0095] Example 3 Synthesis of Compound C: Icariside II (Icariside II), R 1 = Rham, R 2 =H
[0096] Synthesis of 7-O-tert-butyldimethylsilyl dehydroicariin Xiv: compound dehydroicariin A (1 g, 2.7 mmol) with diisopropylethylamine (1.1 ml, 6.0 mmol) at room temperature Dissolve it in 15ml of dichloromethane, keep stirring at 0°C in an ice bath and add dropwise a solution obtained by dissolving tert-butyldimethylsilyl chloride (450mg, 3.0mmol) in 5ml of dichloromethane, after the addition is complete Remove the ice bath, rise to room temperature, stir for 4 hours, add 50ml of 0.5 mol / L hydrochloric acid, separate the dichloromethane phase, extract the water phase with 3×20ml of dichloromethane, combine the organic phases, and wash with saturated sodium chloride solution After washing, drying over anhydrous magnesium sulfate, filtering and concentrating, silica gel column chromatography purification (gradient elution, eluent ratio is acetone:petroleum ether=5:95-10:90) to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com